![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
Catabolism and anabolism of fatty acids via different pathways |
Catabolism of catty acids: Produces acetyl-CoA, produces reducing power NADH, FADH, takes place in mitochondria Anabolism: Requires acetyl-CoA, requires reducing power NADPH, takes place in cytosol in animals, chloroplast in plants |
|
|
Overview of fatty acids synthesis |
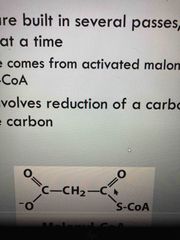
Builds by processing 2 C at a time to yield malonyl CoA (acetate) Each pass requires reduction of a carbonyl C to a methylene C |
|
|
Fatty acid synthesis occurs where NADPH is high |
Cytosol for animals, chloroplast in plants Source of NADPH: from pentose phosphate pathway |
|
|
Malonyl-CoA is formed from Acetyl CoA and bicarbonate |

Yields malonyl CoA Reaction takes acetyl CoA and adds a carboxyl to it by to form actyl-CoA carboxylase (ACC): Has 3 subunits and a biotin that carries CO2. Bicarbonate (H3O) is source of CO2 This occurs in a 2-step reaction: bicarbonate creates carboxyl group. Transferred to biotin ( ATP). 2. Carboxyl group is carried by bio tin to transcarboxylase |
|
|
2 step reaction of ACC |

CO2 binds to biotin and is activated with ATP producing carbamoyl Enzyme undergoes conformational change to carry carbamoyl to transcarboxylase site (are swings around) CO 2 attached to A-CoA and leaves active site |
|
|
Fatty acid synthase (FAS) |

Catalyze a repeating 4 step sequence that elongates the fatty chain 2 C at a time Uses NADPH as an e- donor (reducing agent) Uses 2 enzyme bound SH groups to activate |
|
|
Fatty acid synthase (FAS) I and II |
I: single polypeptide chain in vertebrates, single product palmitate formed, C 15 and 16 are from ACoA used to prime II: made of separate diffusing enzymes in plants and bacteria, makes many branches, lengths |
|
|
Overall FAS type I |
Goal: attach acetate unit from malonyl-CoA to growing chain and then reduce it 4 enzyme catalyzed steps: Condensation: of growing chain Reduction: of carbonyl OH Dehydration: of alcohol trans alkene Reduction: of alkene to alkene |
|
|
Acyl carrier protein (ACP) |

Shuttle acyl group in FAS Has 4’-phosphopantettheine group (flexible arm carries enzymes from one subunit to another) Delivers acetate/malonate to FAS |
|
|
Charging ACP malonyl/acetyl-CoA-ACP transferase (MAT) |

Acetyl group of acetyl-CoA is transferred to KS. ACP passes this acetate and then ACP-SH is charged wi h malonyl 2 thiols must be charged with the correct acyl groups before condensation can begin |
|
|
Condensation of FAS |

Attached 2 C from acetyl group to 2 C from malonyl group Release of CO2 activated malonyl group attachment Creates beta keto intermediate |
|
|
Step 2 FAS: reduction of carbonyl group |

First reduction: NADPH reduces the beta keto intermediate (yellow box) to an alcohol |
|
|
Dehydration step 3 FAS |

OH group from C-2 and H from adjacent CH2 are eliminated and create double bond Catalyzes to DH |
|
|
Second reduction step 4 FAS: reduction of double bond |

NADPH reduces double bond made in previous step to yield saturated alkane |
|
|
Final result of FAS |

4 steps repeat until reaching 16 C where enzyme releases creating palmitate |
|
|
Condensation step 1 |

ACP grabs acetyl COA and attached it to itself |
|
|
step 5 acts as a primer |

ACP grabs acetyl COA and attached it to itself |
|
|
Step 6 |
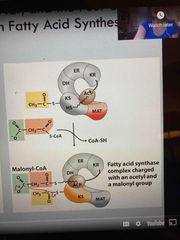
ACP finds another malonyl CoA and attached to KS starting fatty acid |
|
|
Condensation step 1 |

Lose the CO2 group by movement of ACP to the KR area |
|
|
Reduction step 2 |

Begins at KR site and move ACP over to DH domain |
|
|
Dehydration step 3 |

At DH starts dehydration moving ACP to ER section |
|
|
Reduce step 4 |

Remove double bond at ER and then moves ACP group back to KS |
|
|
Reduce step 4 |

Remove double bond at ER and then moves ACP group back to KS |
|
|
Moves ACP back to starting position |
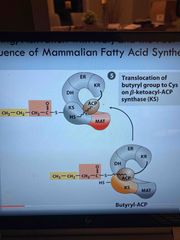
Pic |
|
|
Finally then grabs new malonyl group |
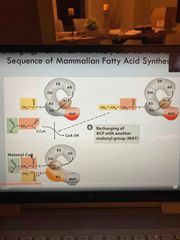
Pic |
|
|
Acetyl COA is made in mitochondria and needs to be transferred to cytosol |
Made from pyruvate oxidation and catabolism of carbons from amino acids This cost 2 ATPs to transfer FAS cost 3 ATPs per 2 C unit |
|
|
FAS is regulated by acetyl CoA carboxylase (ACC) |

ACC is feedback inhibited by palmitoyl but activated by citrate (made in mitochondria and signals extra energy so store fat) ACC is inhibited when energy is needed by glucagon and epinephrine |
|
|
Other modes of regulation in FAS |
Changes in gene expression If extra malonyl CoA, it will inhibit transfer to mitochondria |
|
|
Palmitate can be lengthened to longer chain fatty acids |
Occurs in ER and mitochondria Adds 2-C Stearate (18:0) is the most common product |
|
|
Palmitate can be lengthened to longer chain fatty acids |
Occurs in smooth ER and mitochondria Adds 2-C at a time Stearate (18:0) is the most common product but could be further with additional acetyl groups |
|
|
Can desaturate |

Enzyme desaturase can do this for both palmitate and stearate at position 9 (btw C9 and C10) Also requires cofactors NADPH 4 e- are added: 2 to O and 2 to CH2 bind forming a double bond |
|
|
Plants and humans desaturate |
Humans can’t go past 9 but plants can to 12 and 15 This is why we have to get from our diet. Important for membrane fluidity |
|
|
Plants desaturate by |

Do not oxidize free fatty acids Oxidize fatty acids attached to glycerol Can then be hydrolyzed from glycerol backbone |
|
|
Synthesis of TAGs: Synthesis of backbone of TAGs and phospholipids, then makes phosphadtidic acid (first stage) |
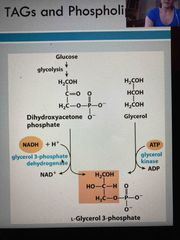
Siphones off dihydroxyacetone phosphate (DHAP) from glycolysis via glycerol 3 dehydrogenase Happens when we have lots energy |
|
|
Synthesis of phosphophatidic acid |

Happens before TAGs Phosphatidic acid is precursor which can be made into either TAGS or glycerophospholipids For TAGS pathway, phosphatidic acid is hydrolyzed by phosphatidic acid phosphatase (lipin) to diacylglycerol Diacylglycerol is then converted to TAGs 2 fatty acids are attached by acyl transferases Releases CoA |
|
|
To form phospholipid and TAGs |

Phosphatidic acid phosphatase (lipin) removes 3-phosphate from acid Then third carbon removed to form Triacylglycerol |
|
|
Regulation of Triacyglycerol |

Insulin results in stimulation of triacylglycerol synthesis Lack of insulin results in failure to synthesize fatty acids from carbs or glucose (can lead to increase ketone body formation) lipolysis ( TAG breakdown) |
|
|
Triacylglycerol breakdown and resynthesis (lipolysis) |

75% of free fatty acids get recycled (reesterified) rather than used for fuel During starvation |
|
|
Biosynthesis of membrane phospholipids |

1. Synthesis of backbone (glycerol or sphingosine), 2. Attachment of fatty acids 3. Addition of hydrophilic head to backbone (through pho Forster linkage) 4. Alteration or exchange of the head group or fatty acids to yield the final phospholipid product Begins with phosphatidic acid or diacylglycerol Attach head gro to C-3 OH group Acid condenses with 2 alcohols and eliminates H2O |
|
|
Activation is required for attaching phospholipid head (2 strategies) |

Needed molecule-Cytidine diphosphate (CDP): contributes the phosphate to connect the head group Strategy 1: CDP is attached to diacylglycerol forming activated phosphatidic acid. Strategy 2 (mammals): CDP is attached to the hydroxyl of the head group |
|
|
Phosphaticdycholine |
Essential in membranes Also called lecithin Derived from phosphatudylethanolamine |
|
|
Phohatidylserine and phosphatidyltic pathways to regulate (NOT ON EXAM) |

Regulation of the two so we don’t have too much through a backward exchange of head groups Savage the choline |
|
|
Synthesis of sphingolipids use similar pathways |

4 stages: 1. synthesis of sphinganine (from palmitoyl and serine) 2. Attachment of fatty acid in amide linkage 3. Desaturation of sphinganine moiety to form N-acylsphingosine (ceramide) 4. Attachment of head group to produce cerebroside or sphingomyelin |
|
|
Cholesterol is built on 5-C unit |

Isoprene |
|
|
Cholesterol synthesis overview |

3 acetates condense to form mevalonate Mevalonate makes activated isoprene 6 isoprenes polymerize to form a 30-C squalene Squalene forms the 4 rings to produce cholesterol |
|
|
Step 1 forming mevalonate from acetate |

2 acetyl-CoAs condense to acetoacetyl-CoA which then condenses with another ACoA to form beta-hydroxy -beta methylglitaryl-CoA (HMG-CoA)—-> catalyzed by acetyl CoA transfer ace and HMG CoA synthase HMG-CoA is a rate limiting step Acetate goes to HMG which is reduced to mevalonate (HMG-CoA reductase) |
|
|
Step 2: conversion os mevalonate to activated isoprenes |

3 phosphates transferred from ATP to mevalonate Decarboxylation and hydrolysis create isoprene Phosphate activated isoprene group |
|
|
Step 3: activated isoprene condensed to form squalene |
Add on isoprenes in steps until get to 30 |
|
|
Step 4: conversion of squalene to 4 ring steroid |

Adds O to end of squalene chain This is where plants and animals are different: product for animals is Lanosterol and for plants is eegosterol |
|
|
Lipoprotein particles |
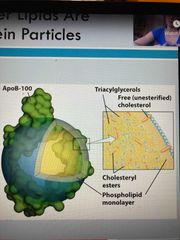
Carry lipids, spherical Surface is made of protein (apolipoprotein) and a Single layer of phospholipids Interior: cholesterol, TAGs, cholesterol esters. All nonpolar so want to keep away from blood |
|
|
4 major classes of Lipoprotein |
Determined by density in centrifuges Chylomicrons VLDL LDL HDL |
|
|
Chylomicrons |

Least dense, very large Contain variety of proteins and TAG Free fatty acid to release fuel in fat, heart, and skeletal muscle When depleted for back to liver via endocytosis by apoE mediated (linked to Alzheimer’s) |
|
|
VertLDL |
Contains TAG and cholesterol ethers in high concentrations Frees fatty acids at adipocytes to recovery them to TAG for storage in lipid droplets Muscle uses TAG for E |
|
|
LDL |

Produced by removal of TAG from VLDL, enriched in cholesterol and ch. ethers Bind to receptors on muscle and adipose tissue Myocytes bad adipocytes take up cholesterol via receptor mediated ebdocytosis |
|
|
HDL |
High density Can convert left over from LDL to esthers for stating other products (bike salts) Pick up cholesterol from cells and return to live to be metabolized (bile salts) |
|
|
Receptor mediated endocytosis |

See pic |
|
|
Lecithin cholesterol Acyl Transferase (LCAT) enzyme |
Present on surface of HDL Allows to recognize cholesterol from LDL and VLDL to combine it with a side chain to convert to cholesterol ethers (starting for bile salts) |
|
|
5 modes of regulation of cholesterol synthesis and transport: first 3 HMG-CoA reductase when dephosphorylated |
Short term regulations AMP dependent prorate in kinase decreases, decrease pathway Glucagon and Epi. Increase down regulate Insulin increase, pathway increases |
|
|
Transcriptional HMG-CoA )longer term) |
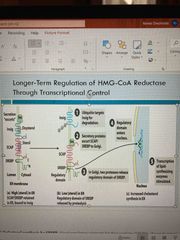
Insulin induced gene protein (Insig) senses cholesterol levels When levels of sterols are high, keeps sterol binding element binding protein (SREBPs) in ER and tags HMG-CoA reductase for degradation with ubiquiton When levels are low, SREBP is cleaved and moved to nucleus for transcription |
|
|
Second regulatory system by LXR mediated transcription |

In liver: liver x receptor |
|
|
Cardiovascular disease causes |

High LDL: inability to clear and begin to build up. Send monocytes which became macrophages and then foam cells which can explode Low HDL cholesterol: picks up cholesterol and carry back to liver so need higher level of these |

