![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
AMPK |

Activated by elevated AMP or decreased ATP by exercise, by sympathetic system or by peptide hormones |
|
|
Some enzymes in the pathway limit metabolites more than others |

Not all regulated enzymes have same effect on the entire pathway Some control flux through pathway while others regulate steady state concentrations in response to changes in flux Ie: hexokinase and phosphofructokinase regulate glycolytic flux: increase hexo to activate glucose and increase PFK-1 catalizes glucose via glycolysis. Hexo has more influence though |
|
|
There are 4 isozymes of hexokinase (different variant of the same enzyme) |

HK 1: expressed in all tissues HK IV (glucokinase): only expressed in the liver, higher Km so more sensitive to high levels of glucose which enables it to clear it from blood for storage when higher levels Also Not inhibited by glucose-6-PO3
|
|
|
HK IV is regulated by sequestration and transcription |

When glucose levels are low in the blood, to shut down HK IV binds to regulatory protein and goes into nucleus To get HK back to cytosol, glucose stimulates receptors GLUT2 HK returns glucose -6-phosphate and fructose-6-phosphate and continues feedback loop |
|
|
Regulation of phosphofructokinase-1 (the conversion of frustose-6-phosphate to fructose 1,6-biphosphate |

Commitment step where adding a phosphate we have committed molecule to glycolysis High levels of AMP lead to this while high levels of ATP inhibit it Citrate also inhibits it due to fatty acid cycle being activated which further confirms the cell has lots of E and we don’t need to make more through glycolysis |
|
|
Regulation of phosphofructokinase-1 and fructose 1,6-bisphosphatase |

For glycolysis: AMP/ADP is high, ATP is low. Activates PFK-1. AMP also inhibits FBpase-1 to prevent gluconeogenesis For gluconeogenesis: AMP is low, ATP is high |
|
|
Fructose 2,6-bisphosphate |

Not a glycolytic intermediate, only a regulator Produced specifically to regulate glycolysis and gluconeogenesis by activating PFK-1 and inhibiting FBPase-2 |
|
|
Glycolysis and gluconeogenesis are regulated by F-2,6-bP |
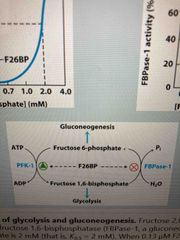
F26BP activates PFK-1 and inhibits FBPase-1 |
|
|
Regulation of F-2,6-bP levels |

A Multifunction singular protein with PRK-2/FBPase-2 combined One side adds PO3 and the other pulls it off When FBPase side is active, stimulates glycolysis and inhibits gluconeogenesis when PKF-2 is active, stimulates gluconeogenesis and inhibits glycolysis |
|
|
Pyruvate kinase regulation |
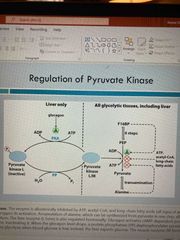
Allosterically activated by fructose-1,6-bispophosphate Allostericallyinhibit by signs of increased E supply (atp, acetyl-CoA, alanine) Inhibited by phosphorylation when glucose depletion occurs (in liver) |
|
|
The flexibility of pyruvate |
Eat a carb rich meal: Pyruvate is first forming ATP through glycolysis When ATP is high, gluconeogenesis is activated. When glycogen storage is full, can convert to acetyl CoA and store fatty acids |
|
|
Glucose can be stored later for use as glycogen |
Glycogen is a branched polymer of alpha 1-4 with alpha 1-6linkages every 12-14 glucose units Occurs mainly in liver and muscle Degraded to glucose for E Can be made from excess blood glucose or recycling glycogenic metabolites like lactate or certain AAs |
|
|
Breakdown of glycogen |

Starts at reducing end Take off a unit of glucose but add a PO3 group to it (glycogen phosphorylase) Product made is a glucose-1-phosphate |
|
|
How it does this with branching |

Glycogen phosphorylase Cuts off at all nonreducing ends until it reaches 4 residues from a branch point but can’t go any further Debranching enzyme can then move 3 of the 4 molecules to the end of another branch Then goes in and cleaves off last glucose molecule which becomes a free molecule |
|
|
Glucose-1-phosphate (single glucose molecule) must be isomerized to glucose-6-phosphate for metabolism |

Phosophoglucomutase performs this rxn |
|
|
To transfer glucose-6-phosphate out of the liver… |

Glucose-6-phosphatase is sequestered in the ER lumen Dephosphorylates glucose-6-phosphate in the liver Uses concentration gradient to control flux out of liver |
|
|
Glycogen synthesis from glucose in multiple steps |
Needs more enzymes Must be phosphorylated, labeled with UDP, and added to glycogen Multiple steps allow for multiple regulation points |
|
|
Gloves synthesis |

Use enzyme glycogen synthase Glucose molecule is attached to UDP glucose (uracil w PO3) Then adds to nonreducing end |
|
|
UDP glucose (uradene) and how it is formed |

See pic |
|
|
Synthesis of branches in glycogen |

After 6 or 7 glucose molecules, glycogen-branching enzyme cleaves and reattached 4 glucose molecules towards core Product is one 4 molecule chain and one 1-6 molecule chain with nonreducing ends |
|
|
Glycogenin molecule (core) |
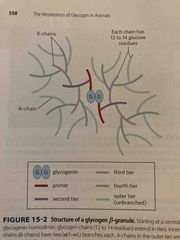
Creates new glucose chains using UDP starting with a primer of 6 glucose molecules Then more chains build out from there |
|
|
Layout of glycogen synthesis and degradation |

See pic |
|
|
Control of glycogen breakdown |

Flu gig in/epinephrine signaling pathway starts phosphorylation cascade via cAMP and activates glycogen phosphorylase Glycogen phosphorylase cleaves glucose residues off creating glucose-1-phosphate |
|
|
Epinephrine & glucagon stimulate breakdown of glycogen |
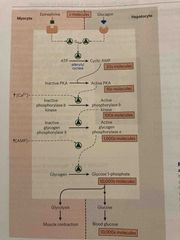
See pic |
|
|
Control of glycogen synthesis |

Insulin-signaling pathway increases glucose into muscle, stims hexokinase, and activates glycogen synthase Makes glycogen for E storage |
|
|
Glycogen synthase is controlled by phosphorylation |

When phosphorylated, Inhibited So when we want glycogen, add PO3 groups |
|
|
Glycogen-targeting protein (GM) |

Can be phosphorylated by insulin or epinephrine (opposite rxn) Family of proteins |
|
|
Summary graph in book |

Page 70 |
|
|
In liver pathways |

Page 570 |

