![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
Chemoosmotic theory |
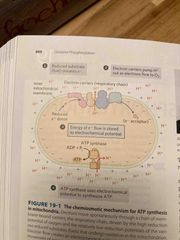
E to convert ADP to ATP is provided by a flow of e- down the electrochemical gradient The E released is used to transport protons against the gradient |
|
|
E coupling requires membranes |
The proton gradient needed for ATP synthesis is across the membrane: Plasma membrane in bacteria, inner membrane of mitochondria, and thylakoid membrane in chloroplasts Membrane must contain proteins that couple the downhill flow of e- with the uphill flow of H+ |
|
|
Structure of mitochondria: double membrane leads to 4 compartments |
1. Outer membrane: porous 2. Intermembrane space : higher H+ concentration (low pH) 3. Inner membrane: mostly impermeable w H+ gradient across it, location of ETC complexes, cristae increase surface area 4. Matrix: location of TCa cycle, part of lipid and AA metabolism, lower H+ concentration (high pH) |
|
|
Electron transport chain uses a series of e- carriers |
Multiple redox centers at each complex: Flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), cytochromes a, b, c, iron-sulfur cluster Order of transfer depends on reduction potential Ends with O being reduced to water |
|
|
FMN and FAD : e- funnels |
Can bind to proteins to funnel and distribute e- Accept 2 e- from carrier and donate 1 e- at a time |
|
|
Cytochromes |
One-electron carriers Do so by iron coordinating porphoryn ring a, b, c differ by ring additions |
|
|
Iron sulfur clusters |

One-electron carriers Coordinating by cysteines in proteins Contain equal number of iron and sulfur atoms |
|
|
Coenzyme Q (ubiquinone) |
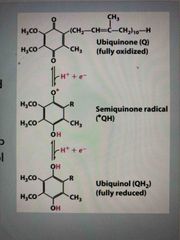
Lipid soluble, carries 1 or 2 e- If accepts 2 e-, called ubiquinol which can freely diffuse carrying e- from one side of membrane to other Mobile carrier and transports e- from complex I, II, and III. |
|
|
Complex I: ubiquinone oxireductase |

Largest macro-molecule in mammals NADH binding site on matrix side FMN accepts 2 e- from NADH Several iron sulfur centers pass one e- at a time toward the Q binding site |
|
|
It is a proton pump |
Transfer 2 e- from NADH to Q is accompanied by a transfer of H+ from the matrix (N) to the intermembrane space (P). 4 H+ per NADH transferred Proteins are transported by protein wires |
|
|
Complex II: Succinate dehydrogenase |

FAD accepts 2 e- from succinate e- are passed one at a time via iron-sulfur centers to Q becoming reduced QH2 Does not transport H+ Succinate dehydrogenase is a single enzyme with dual roles: converting fumarate in CAC and capture and donate e- in ETC |
|
|
Complex III: cytochrome c oxidoreductase |

Creates QH2 and in complex I and II so now use it to reduce 2 molecules of cytochrome c (bc they can only hold one each) Done through iron clusters cytochrome b and c Results in translocation of 4 additional H+ to intermembrane space |
|
|
The Q cycle |

4 H+ are transported across membrane per 2 e- that reach cyt c (2 of 4 H+ come from QH2) Occurs in 2 stages 2 molecules of QH2 became oxidized releasing H+ in the intermembrane One molecule becomes re-reduced totaling 4 H+ per Q |
|
|
Cytochrome c |
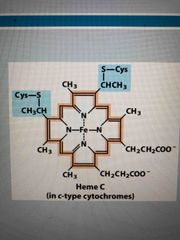
The 2nd mobile e- carrier Soluble heme (Fe) so able to move through membrane Carries single e- |
|
|
Complex IV: cytochrome oxidase |

Membrane protein with 13 subunits Contains 2 heme groups Contains copper ions: CuA: 2 ions that accept e- from cyt c CuB: bonded to heme forming center that transfers 4 e- to O |
|
|
e- flow through complex IV |

4 e- are used to reduce 1 O molecule into 2 water molecules 4 H+ are picked up from matrix in this process 4 additional H+ are passed from matrix to intermembrane space |
|
|
Summary of e- flow |

See pic. Also in book on 672 |
|
|
Summary of how many H+ we get |

See pic |
|
|
Another helpful image of transport chain |

See pic and on page 675 |
|
|
Damage to biological macromolecules: free radicals |

Q can be leaky and is unstable Single e- becomes free radical and can become H2O2 Glutathione helps to convert to water |
|
|
Proton-motive force m: 3 ways that create the electrochemical proton gradient |

1. Actively transporting H+ across membrane (complex I and IV) 2. Chemically removing H+ from matrix (reduction of Q and O) 3. Releasing H+ into intermembrane space (oxidation of QH) |

