![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Ligand binding |
Specificity of ligand sand binding sites Coupled to conformational changes (induced fit) Conformational changes can affect others in one subunit (cooperativity) Interactions can be regulated |
|
|
Functions of globular proteins |
Storage of ions and molecules (myoglobin, ferritin) Transport of ions/molecules (hemoglobin, serotonin transport) Defense against pathogens (antibodies, cytokines) Muscle contraction (actin, myosin) Biological catalysis (chymotrypsin, lysozyme) |
|
|
Molecule that binds to a protein is a |
Ligand The region where it binds is binding site Binds via non covalent interactions that dictate protein structure |
|
|
Quantitive description for binding |
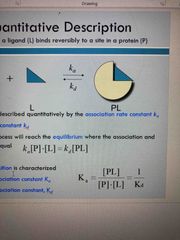
Ka: association rate constant Kd: dissociation rate constant After some time process will reach equilibrium |
|
|
Fraction of occupied bound sites |

Equilibrium dissociation constant The fraction of bound sites depends on free ligand concentration and Kd |
|
|
Example of oxygen binding to myoglobin |
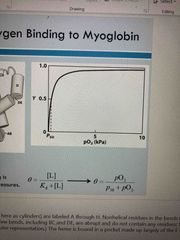
See pic |
|
|
Specificity |
Lock and key model for certain ligands Based on: Size shape charge and hydrophobic/hydrophilic These characteristics are performed according to fisher |
|
|
Induced fit |

Changes occur upon ligand binding Allows for tighter binding of ligand and high affinity for different ligands |
|
|
Globing are oxygen binding proteins |

Oxygen molecule is captured with heme that is protein bound Myoglobin (storage in muscles) and hemoglobin (transfer of oxygen) |
|
|
Binding of CO |
Similar size and shape to O2and can fit the same binding site. Bonds 20,000 times better than O2 due to lone pair being donated |
|
|
Chromophore |
The heme group absorbs both in visible range and ultraviolet Deoxygenated blood appears purple and oxy hemoglobin blood is red |
|
|
Could myoglobin transport O2? |

In lungs-13 kPa, binds oxygen In tissues-4 kPa, will not release it For effective transport affinity must vary: bind in lungs where pO2 is high and release in tissues where pO2 is low |
|
|
Cooperativity |
Positive: increases affinity after first binding Negative: reduces affinity after first binding |
|
|
Concerted v sequential cooperativity |

See pic |
|
|
Allosteric regulation |
Cooperativity is an example of this Homotropic: normal ligand is the allosteric regulator Heterotropic: a different ligand affects binding of normal ligand |
|
|
Subunit interactions in hemoglobin |
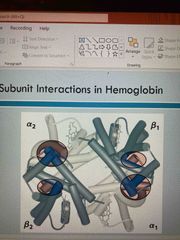
See pic Alpha 1 and 4 Beta 1 and 2 |
|
|
R and T states of hemoglobin |

R: relaxed state, less interactions, more flexible, higher affinity for O2 T: tense, more interactions, more stable, low affinity for O2 O2 binding triggers T to R conformational change Involves breaking ion pairs btw alpha 1 and beta 2 |
|
|
R and T states |

See pic |
|
|
pH effect on binding: Bohr effect |
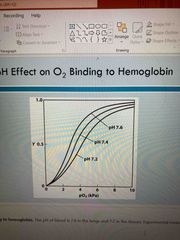
ph in lungs, 7.6 and tissues, 7.2 Lower in tissues due to active metabolizing generating H+ H+ binds to Hb and stabilizes T state which leads to release of O2 this increasing efficiency of transport |
|
|
CO2 export |

Produced by metabolism in tissues 15-20% is exported in the form of carbonate on amino terminal residues of each polypeptide of Hb This process yields a proton that contributes to Bohr effect |
|
|
2, 3 bisphoaphoglyverate regulates O2 binding |
Stabilizes T state, negative charge, bind to positive charge of Hb Allows release in tissue at high altitudes |
|
|
Sickle cell mutation |
New valine side chain can bind to different Hb molecule to form a strand similar to amyloid IgE if proteins |
|
|
Antigens |
Stimulate production of antibodies Recognized as foreign by immune system Coats proteins of bacteria and viruses Surface carbohydrates of cells or viruses |
|
|
Antibodies |
Proteins that are produced by B cells and that specifically bind to antigens Binding will mark antigen for destruction Binds to small region of antigen (epitope) One antigen can have several epitopes |
|
|
Immunoglobulin G |
Antibody with 2 heavy chains and 2 light chains (composed of variable and constant domains) Variable chains make up antigen binding sites (2 per antibody) which confers antigen specificity |
|
|
Antigens bind via induced fit |
Antigen binding causes significant structural changes to that antibody |
|
|
Review of muscle |
See pic |
|
|
Myofibrils contain thick and thin filaments |
Thick: myosin Thin: actin |
|
|
Atp and muscle |
See pic |
|
|
Muscle contraction |
Ca binds to Troponin causing it to shift on tropomyosin exposing myosin binding sites Myosin heads bind to sites creating the power stroke |

