![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
|
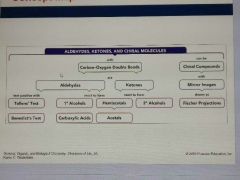
What feature of aldehydes and ketones strongly influences their physical and chemical properties? |
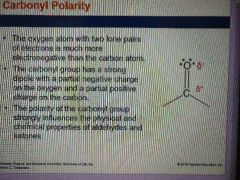
Carbonyl Polarities |
|
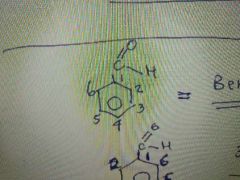
What is the name of this molecule? |
Benzaldehyde |
|
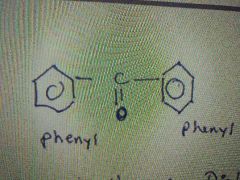
What's the name of this molecule? |
Benzophenone |
|
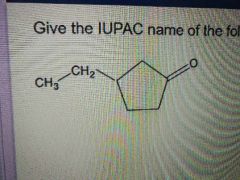
What's the name of this molecule? |
3-ethylcyclopentanone |
|
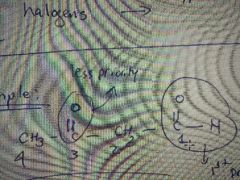
What's the name of this molecule? |
3-oxo-butanal |
|
|
With what conditions are aldehydes and ketones gases and liquids? |
With carbon chains 1 - 2 atoms long and 3 - 10 atoms long respectively. |
|
|
What functional group does Benzene belong to? |
Phenyl group |
|
|
Why do aldehydes and ketones have higher boiling points than alkanes and ethers of similar mass? |
Dipole-dipole interactions between carbonyl groups hold aldehydes and ketones together |
|
|
Why do alcohols have a higher boiling point than aldehydes and ketones of similar mass? |
Alcohols can form hydrogen bonds with each other and aldehydes and ketones cannot |
|
|
At what point are aldehydes and ketones soluble, slightly soluble, and non soluble? |
At one to four, five, and six or more carbon atoms respectively |
|
|
What do aldehydes and ketones oxidize to form? |
Aldehydes form carboxylic acids, ketones do not undergo oxidization. |
|
|
What is a result of the addition of an alcohol to an aldehyde or ketone in the presence of an acid catalyst? |
A hemiacetal |
|
|
What type of molecule contains two alkoxy groups on the same carbon atom? |
Acetals |
|
|
Of the two, which are more reactive? Aldehyde or ketones? |
Aldehydes, because their carbonyl carbon is more positive |
|
|
Which aldehydes are more often referred to by their common names? |
Aldehydes with carbon chains of 1 to 4 carbons. |
|
|
Are numbers needed for naming both aldehyde and ketone groups? |
Only for ketone groups |
|
|
What are the common names for methanal and ethanal? |
Formaldehyde and acetaldehyde. |
|
|
Why are aldehydes and ketones soluble at all? |
The oxygen in the carbonyl group forms a hydrogen bond with the water's hydrogen atom. |
|
|
What do aldehydes oxidized to form? |
Carboxylic acids |
|
|
Why can't ketones undergo oxidization? |
Because there is no hydrogen atom connected to the carbonyl group to be reduced/oxidized |
|
|
What is Tollen's reagent? |
A solution of a positive silver ion and ammonia (AgNO3) |
|
|
What is the purpose of tollens test? |
To differentiate between oxidizable aldehydes and inoxidizable ketones. |
|
|
What is the purpose of Benedict's test? |
The differentiate between compounds with an aldehyde functional group and an adjacent hydroxyl group and simple aldehydes and ketones. |
|
|
What is Benedict's solution? |
CuSO4 (containing a Cu²+ ion) |
|
|
What is the product of Benedict's solution and and aldehyde with an adjacent hydroxyl group? |
A brick-red solid of Cu2O. |
|
|
What is the product of a positive tollens test? |
Reduced silver and an oxidized aldehyde, forming a silver mirror on the inside of the container. |
|
|
By what are aldehydes and ketones reduced? |
Hydrogen (H2) or sodium borohydride (NaBH4) and a catalyst such as nickel platinum or palladium. |
|
|
What are the process and products of reduction of aldehydes and ketones? |
An addition of hydrogen reduces carbon oxygen bonds. The products of reduction of aldehydes and ketones are primary and secondary alcohols respectively. |
|
|
What is the product of butanal of Tollens' reagent? |
Butanoic acid and silver |
|
|
What is the product of the reduction of cyclohexanone by hydrogen? |
Cyclohexanol |
|
|
With an abundance of alcohol, what will a hemiacetal become? |
The unstable hemiacetal will react with a second molecule of alcohol to form acetal and water. |
|
|
What is the difference in carbonyl groups between aldehydes and ketones? |
Polarity, the carbonyl group in the more reactive aldehyde is more positive. |
|
|
Is hemiacetal and acetal formation reversible? |

Yes. |
|
|
Under what conditions do cyclic hemiacetals form? |
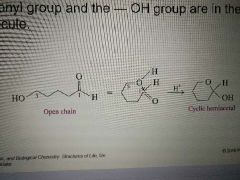
When the carbonyl group and the -OH group are in the same molecule. |
|
|
What reaction explains how sugar molecules can link together and form disaccharides and polysaccharides? |
The addition of alcohols to cyclic hemiacetals to form cyclic acetals. |
|
|
What is maltose and what are its constituents? |
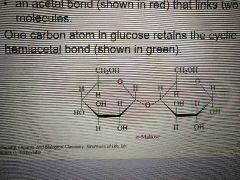
A disaccharide consisting of two glucose molecules linked together by an acetal bond. |
|
|
What is the condensed structural formula of the acetal formed by adding CH3OH to butanal? |

|
|
|
What does it mean for a carbon atom to be chiral? |
The carbon atom must have 4 different atoms or groups. |
|
|
What is a stereoisomer? |
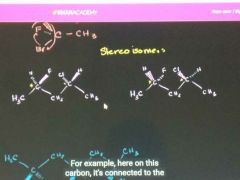
An arrangement of a molecule in which only the three-dimensional image looks different than the original.. |
|
|
What is a Fischer projection? |
A two-dimensional representation of a molecule, placing the most oxidized group at the top, using vertical lines for bonds that go back, and horizontal lines for bonds that go forward |
|
|
What is an enantiomer? |
A stereoisomer that cannot be superimposed with its original |
|
|
What senses are responsive to the chirality of molecules? |
Smell and taste |
|
|
Remember this |

