![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
|
General form |
A- carbonyl group and -H on same carbon K- 2 carbonyl groups attached -Formaldehyde has 2 -H attached -Nucleophilic addition occurs at carbonyl -Higher BP than hydrocarbons of same weight (polarity of carbonyl(s)) -Lower BP than corresponding alcohols (no -H bond between molecules) |
|
|
Nomenclature Aldehydes |
IUPAC replace -e with -al methanal (formaldehyde), ethanal (acetaldehyde), pheynlethanal (phenylacetaldehyde) Carbaldehyde: -CHO is attached to a ring system cyclohexanecarbaldehyde, benzaldehyde -COH as prefix is formyl or methanoyl group 2-Methanoylbenzoic acid (o-formylbenzoic acid) |
|
|
Nomenclature Ketones |
IUPAC replace -e with -one Number with -CO at lowest possible -COCH3 as prefix is ethanoyl or acetyl group (Ac) -COR as prefix is alkanoyl or acyl group 4-Ethanoylbenzenesulfonic acid (p-acetylbenzenesulfonic acid) |
|
|
Aldehydes by Oxidation of 1o Alcohols Swern |

oxidation state lies between that of primary alcohol and carboxylic acid |
|
|
Aldehydes by Oxidation of 1o Alcohols PCC |
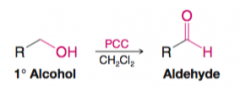
pyridinium chlorochromate |

