![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
87 Cards in this Set
- Front
- Back
|
Aldehydes & ketones in solution are considerably __ basic than alcohols
|
less (conjugate acids are more acidic because of solvent effect: in gas phase, aldehydes & ketones more basic, in solution, solvation of protonated alcohol by hydrogen bonding is evidently so effective that it outweighs resonance stabilization of protonated aldehyde/ketone
|
|
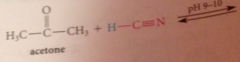
Addition
|
|
|
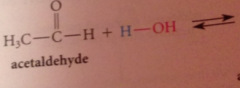
In all carbonyl-addition reactions, the more electropositive species adds to
|
the carbonyl oxygen, & the more electronegative species adds to the carbonyl carbon
|
|
|
Nucleophilic carbonyl addition
|

Reaction of a nucleophile @ carbonyl carbon: i.e. cyanide ion donates e to carbonyl C of ald/ket & carbonyl o accepts displaced e pair & assumes neg charge, which is protonated by h2o or hcn
|
|
|
Nucleophilic carbonyl addition occurs even though the C-O bond is stronger than the C-C pi bond because
|
the unshared e pair & neg charge formed in the mech is transferred to the electroneg O & same rxn of alkene would place unshared pair & neg charge on C
|
|
|
Can a nucleophile add to the carbonyl oxygen?
|
No: nucs always react w carbonyl groups @ the carbonyl C
|
|
|
How does the carbonyl carbon change hybridization when attacked?
|
sp2 to sp3, trigonal planar to tetrahedral: angle compresses, groups bound to the carbonyl become closer together
|
|
|
Why does the addition geometry occur?
|

The bonding pi MO of the carbonyl group is fully occupied w 2 e and cannot have any more so e pair of nuc interacts w LUMO: antibonding MO w lobes above & below & the nuc must begin bonding w carbonyl from direction along which lUMO is conc
|
|
|
When the antibondnig pi* MO is filled
|
the C=O bond is weakened and breaks
|
|
|
Second mechanism for carbonyl addition
|

analogous to mech for addition of acids to alkenes: protonation of carbonyl O
|
|
|
Mechanism 2.2 carbonyl addition
|
Loss of a proton to solvent (weak base H2O can react because protonated O makes a strong Lewis acid)
|
|
|
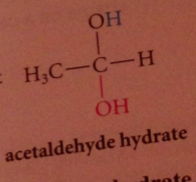
Does hydration of aldehydes & ketones occur in neutral & basic solutions?
|
Yes
|
|
|
Mechanism 2.2 direction of approach
|
above or below bc of shape of LUMO
|
|
|
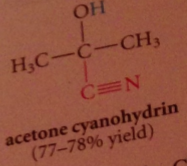
Hydration & cyanohydrin formation are both __
|
reversible rxns
|
|
|
Whether the equilibrium favors addition product or carbonyl cmpd depends on
|
the structure of the carbonyl cmpd
|
|
|
Addition is more favorable for aldehydes or ketones?
|
Aldehydes
|
|
|
Electronegative groups near the carbonyl C make addition ___ favorable
|
more
|
|
|
Groups that donate e by resonance to the carbonyl C make addition ___ favorable
|
less
|
|
|
The equilibria for all addition rxns show similar effects of structure important bc
|
the reactivities of carbonyl cmpds follow similar trends
|
|
|
Reason for the effect of structure on carbonyl addition?
|
The relative stabilities of the carbonyl compound & the addition product govern delta G for addition: the primary effect on the hydration equilibrium is the difference in the stabilities of the carbonyl compounds - Added stability in the carbonyl cmpd inc delta G & dec equilibrium constant for formation of an addition product
|
|
|
Carbonyl cmpds are stabilized bc
|
one resonance structure reflects polarity of carbonyl group w characteristics of a carbocation so anything that stabilizes carbocations also stabilizes carbonyl cmpds
|
|
|
Why are ketones more stable than aldehydes?
|
Alkyl groups stabilize carbocations, so the equilibria for additions are less favorable
|
|
|
Formaldehyde has 2 hydrogens so its equilibrium constant for hydration is
|
large
|
|
|
Electronegative groups such as halogens ___ carbocations
|
destabilize by polar effect & carbonyl cmpds, so make equilibria for addition more favorable
|
|
|
Groups that are conjugated w the carbonyl group such as phenyl of benzaldehyde
|

stabilize carbocations by resonance & hence stabilize carbonyl cmpds: cannot occur in hydrate bc carbonyl no longer present
|
|
|
Aryl aldehydes & ketones have ___ hydration equilibria
|
relatively unfavorable
|
|
|
Steric effect of carbonyl addition
|
As size of groups bound to carbonyl carbon inc, VDW in addition cmpds inc in importance
|
|
|
Cmpds w favorable addition equilibria tend to react
|
most rapidly in addition rxns
|
|
|
Aldehydes are generally ___ than ketones in addition rxns
|
more reactive
|
|
|
Formaldehyde is ___ reactive than many other simple aldehydes
|
more
|
|
|
Reason for parallel trends in rates and equilibria
|
Transition states for addition rxns resemble addition products
|
|
|
LiAlH4 is a source of
|
a hydride ion (very basic) & bc H is more electroneg than Al, the Al-H bonds of the -AlH3 ion carry a substantial fraction of the neg charge
|
|
|
LiAlH4 must be used in __ solvents
|

dry, such as anhydrous ether and THF
|
|
|
The rxn of LiAlH4 w aldehydes & ketones involves nuc rxn of hydride @ __ and the lithium ion acts as a ___ by ___
|
carbonyl C - Lewis acid catalyst by coordinating to the carbonyl oxygen
|
|
|
The addition product (an alkoxide salt) can react w AlH3 & resulting product can serve as
|

a source of hydride: similar process at each stage of reduction until all hydrides consumed
|
|
|
After the reduction is complete, the alcohol prod exists as
|
an alkoxide addition cmpd w aluminum, converted separately into alcohol (proton from HCl or aq. NH4Cl)
|
|
|
Sodium ion is a __ LA than lithium ion
|
weaker, so NaBH4 reductions carried out in protic solvents such as alcohols
|
|
|
Hydrogen bonding btwn alcohol solvent & carbonyl group serves as
|
weak acid catalysis that activates the carbonyl group
|
|
|
Are all four hydride equivalents of NaBH4 active in reduction?
|
Yes
|
|

|
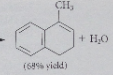
|
|
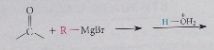
The net effect of the Grignard reaction followed by protonolysis is
|
addition of R-H (R=alkyl or aryl group) across the C=O double bond
|
|
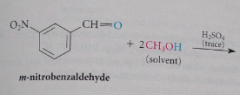
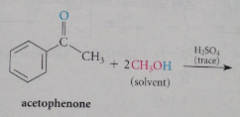
When an aldehyde or ketone reacts w a large excess of an alcohol in the presence of a trace of strong acid
|
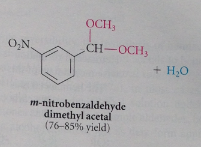
an acetal is formed
|
|
|

|
|
|
acetal
|
cmpd in which 2 ether O are bound to same C (ethers of carbonyl hydrates, or gem-diols)
|
|
|
How many equivalents of alcohol are consumed in each acetal-forming reaction?
|
2
|
|
|
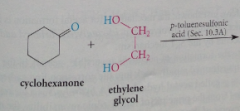
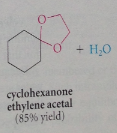
One equivalent of a 1,2 or 1,3-diol can react to form a
|
cyclic acetal in which the acetal group is part of a 5 or 6 membered ring, respectively
|
|
|
|
|
|
Is the formation of acetals reversible?
|
Yes
|
|
|
The reaction is driven to the right by
|
the use of excess alcohol as the solvent or the removal of the water by-product, or both
|
|
|
benzene-water azeotrope
|
mixture of benzene and water that has a lower boiling point than either benzene or water alone
|
|
|
Mechanism for acetal formation
|
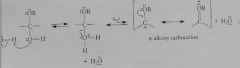
acid-catalyzed addition of the alcohol to the carbonyl group to give a hemiacetal, which reacts when OH is protonated & water lost to give relatively stable carbocation, an a-alkoxy carbocation
|
|
|
hemiacetal
|
compound with an OR and OH group on same carbon
|
|
|
Mechanism 2
|
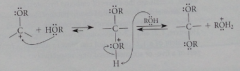
Loss of water from the hemiacetal is an Sn1 rxn -> nuc rxn of an alcohol w the cation & deprotonation of nuc O
|
|
|
Mechanism for acetal formation is really combination of
|
acid-catalyzed carbonyl addition followed by substitution by SN1 mechanism
|
|
|
Acetal hydrolysis
|
acetals in presence of acid & excess h2o transformed rapidly back into corresponding carbonyl cmpds & alcohols
|
|
|
The formation of hemiacetals is catalyzed by
|
acids and bases
|
|
|
the conversion of hemiacetals into acetals is catalyzed
|
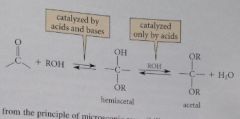
only by acids
|
|
|
In what solutions are acetals stable?
|
basic and neutral
|
|
|
Hemiacetals in most cases cannot be isolated because
|
they react further to yield acetals (in alcohol solution under acidic conditions) or decompose to aldehydes or ketones and an alcohol
|
|
|
Do simple aldehydes form appreciable amts of hemiacetals in alcohol solution?
|
Yes, just as they form appreciable amounts of hydrates in H2O
|
|
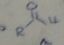
hydroxy aldehydes spontaneously form
|
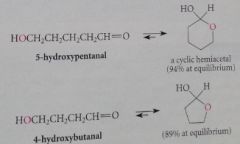
five and six membered cyclic hemiacetals, and most are stable cmpds that can be isolated
|
|
|
Intramolecular rxns are faster and..
|
favored thermodynamically (have larger equilibrium constants)
|
|
|
Protecting group
|
chemical disguise so that a reagent reacts w one mlc and not another: most common are acetals for aldehydes & ketones
|
|
|
Acetals are commonly used to protect the carbonyl groups of aldehydes & ketones from
|
basic, nuc reagents
|
|
|
Once the protection is no longer needed
|
The acetal protecting group is removed, carbonyl re-exposed by treatment w dilute aq. acid
|
|
|
Primary amine
|
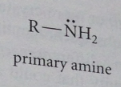
organic derivative of ammonia in which only one ammonia hydrogen replaced by an alkyl or aryl group
|
|
|
imine
|
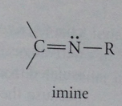
nitrogen analog of an aldehyde or ketone in which C=O group replaced by C=NR group
|
|
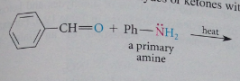
|
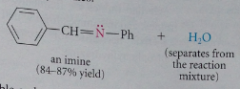
reversible, generally w acid or base catalysis or w heat, typically driven to completion by ppt of imine removal of H2O or both
|
|
|
Mechanism imine formation
|
nuc addition to carbonyl to give unstable carbinolamine
|
|
|
carbinolamine
|
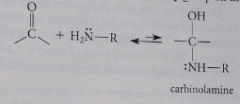
cmpd w amine group -NH2, -NHR or NR2 & hydroxy group on same C
|
|
|
Mechanism imine formation 2
|
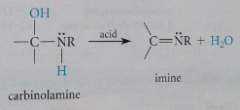
carbinolamine undergoes acid-catalyzed dehydration to form imines, faster than dehydration of ordinary alcohol
|
|
|
Why is imine formation catalyzed by acids?
|

The dehydration is typically the rate-limiting step, but if acid conc is too high --> the protonated (pulls equilibrium to left) amine (basic) cannot act as a nuc
|
|
|
Uses of imines
|
preparation of amines, characterization of aldehydes & ketones
|
|
|
derivatives
|
crystalline cmpds: basis for ID of cmpd when isolated from a source or from a diff. rxn
|
|
|
Why is it important to prepare derivatives?
|
If two compounds have very similar mp or bp
|
|
|
secondary amine
|
R2NH
|
|
|
Enamine
|
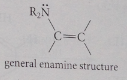
amine nitrogen bound to a carbon that is part of a db
|
|
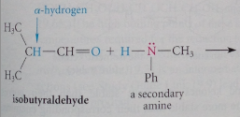
provided that the carbonyl has an a-hydrogen, formation of a ___ occurs when a secondary amine reacts with an aldehyde or ketone
|
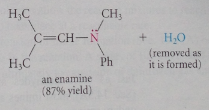
|
|
Is this process reversible?
|
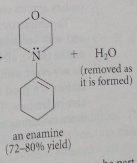
Yes (formation of an enamine) & must be driven to completion by the removal of one of reaction products (usually H2O)
|
|
|
In what do enamines revert to the corresponding carbonyl cmpds?
|
aqueous acid
|
|
|
Mechanism of enamine formation
|
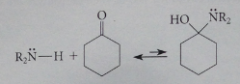
Nuc addition: carbinolamine w no hydrogen on nitrogen so imine formation cannot occur
|
|
|
Enamine mech 2
|
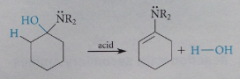
dehydration of carbinolamine: loss of H from an adjacent C
|
|
|
Why don't primary amines react w aldehydes or ketones to form enamines rather than imines?
|

The enamines bear the same relationship to imines that enols bear to ketones
|
|
|
What is more stable, an imine or enamine?
|
Imine
|
|
|
tertiary amine
|
R3N
|
|
|
Do tertiary amines react w aldehydes or ketones to form stable derivatives?
|
no
|
|
|
Tertiary amines are good nuc but have no ____ so cannot form carbinolamines
|
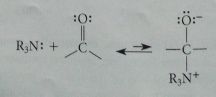
NH hydrogens - adducts w aldehydes & ketones are unstable, can only break down to starting materials
|

