![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Henry's Law
|
- solubility of gas depends on its partial pressure above the solution
- partial pressure of a gas in solution is equal to partial pressure of that gas in gas phase with which the solution is in equilibrium - amount of dissolved gas dependent directly on partial pressure because blood temperature constant - at equilibrium plasma and alveolar PO2 is 100 mmHg - at 37C, plasma will contain about 0.3 mL O2/100 mL of fluid |
|
|
oxygen dissolved
|
- can't dissolve enough oxygen in blood
- pigments help with this |
|
|
four chemical categories of respiratory pigments
|
- hemoglobin
- hemocyanin - hemerythrin - chlorocruorin |
|
|
hemocyanin
|
- second most common of respiratory pigments in animals
- no heme iron or porphyrin ring - copper binds directly to protein - contain copper - mostly clear but turn bright blue when oxygenated - always dissolved in blood plasma/hemolymph - multiple subunits |
|
|
two types of hemocyanin
|
- arthropod hemocyanin: crabs, lobsters, crayfish, horseshoe crabs, spiders and some other arthropods
- mollusk hemocyanin: squids, octopus, many snails, and some other mollusks |
|
|
chlorocruorins
|
- close chemical similarities to hemoglobin
- iron porphyrin ring but differ in heme - sometimes called green hemoglobin - only in four families of marine annelid worms - always dissolved in blood plasma - heme like group |
|
|
hemerythrins
|
- non-heme iron containing respiratory pigments
- located intracellularly in blood or coelomic cells - oxygen binding site is binuclear iron center - iron atoms are coordinated to protein through the carboxylate side chains of glutamate and asparate, and five histidine residues - colorless when deoxygenated but reddish violet when oxygenated - heme like group: 2 irons |
|
|
myoglobin
|
- single-chain globular protein of 153 amino acids
- contain a heme - primary oxygen-carrying pigment of muscle tissues - doesn't exhibit cooperative binding oxygen - binding of oxygen is unaffected by oxygen pressure in surrounding tissue - often cited as having "instant binding tenacity" to oxygen - hyperbolic oxygen dissociation curve - higher affinity for oxygen - doesn't have to change its shape |
|
|
hemoglobin
|
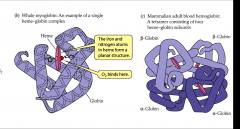
- most widespread respiratory pigment
- multiple forms of hemoglobin differ not in heme but in globin portion - protein portion changes through lifetime - 4 polypeptides - 2 alpha ad 2 beta chains - each chain has a heme group |
|
|
human developmental changes
|
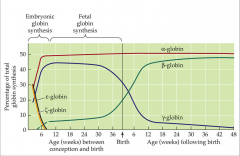
- change in types of globin synthesized for blood hemoglobins
|
|
|
animal size and O2 affinity
|
- smaller bodies species tend to exhibit lower oxygen affinity and thus higher P50 than larger bodied ones
-maybe due to relationship between weight specific metabolic rated and body size - lower affinity Hg in smaller species unload oxygen to tissues more readily due to lower affinity - higher the P50, the lower the affinity |
|
|
factors affecting loading and unloading to hemoglobin
|
- cooperativity
- pH effects - haldane - temperature - 2,3 DPG |
|
|
oxygen equilibrium curve
|
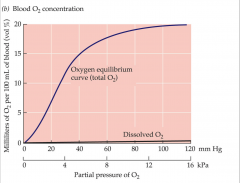
- 4 polypeptides of oxygen bind at different rates
- diosteric change from binding of 1 allows for 2 and 3 to bind faster - 2 and 3 binding help the 4 - total saturation include Hg and plasma - plateau means there is wide range of blood oxygen partial pressures that is sufficient to saturate blood hemoglobin |
|
|
oxygen delivery by human blood at rest and during exercise
|
- O2 released to tissues by each 100 mL of blood
- drop in blood [O2] as it passes through tissues - deoxygenation in tissues is increased - as O2 partial pressure of blood falls, less of a drop is required to cause unloading of 5% vol O2 |
|
|
loading and unloading are cooperative
|
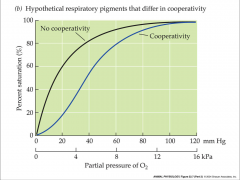
- shape of oxygen equilibrium curve depends on oxygen binding site cooperativity
|
|
|
- O2 affinity of hemoglobin
|
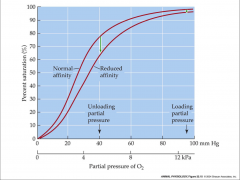
- decrease can aid O2 delivery to systemic tissues
- reduced affinity shows decrease in percent saturation |
|
|
Bohr effect
|
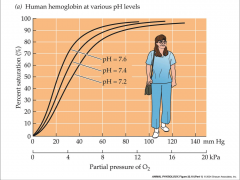
- CO2 blown off as you move away from lungs
- more CO2 in tissue - more basic = higher affinity - more acidic = lower affinity - tissue has lower pH then lungs - tissues experience a lower affinity - typically enhances O2 delivery - oxygen unloading is greatest |
|
|
shift to right in oxygen equilibrium curve
|
- reflects a decrease in O2 affinity
- oxygen partial pressure needed to saturate is higher - P50 is higher |
|
|
root effect
|
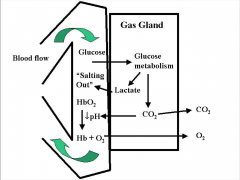
- up or down affect
- maximum saturation you can get due to pH - used to inflate swim bladders - lactate acid can change pH - lowered pH and increased CO2 decrease O2 carrying capacity - acidification lowers oxygen-carrying capacity of hemoglobin |
|
|
temperature
|
- cause right to left shift, up and down shift
- lower affinity for oxygen at higher temperatures - increase in temperature typically causes a decrease in O2 affinity |
|
|
2,3 - DPG
|
- normal p50 of human hemoglobin within RBC depends on normal intracellular concentration of 2,3-DPG
|
|
|
concentrations of specific inorganic ions
|
- can allosterically modulate O2 affinity
- Cl- hemoglobin - bicarbonate (HCO3-) in crocodilians - Ca2+ and Mg2+ in hemocyanin |
|
|
fetal hemoglobin
|
- higher affinity than maternal
- can't bind to 2,3-DPG - loads in fetal and unloads in maternal |
|
|
CO2 transport
|
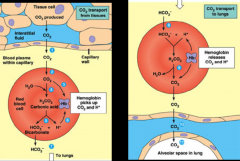
- most CO2 in blood is in form of bicarbonate
- extent of bicarbonate formation depends on blood buffers - blood of mammals effective in buffering H+ generation from CO2 - buffer groups on hemoglobin are major buffers - terminal amino group and imidazole groups found where histadine occurs |
|
|
imidazole groups
|
- dominate buffer
- very efficient - allow large amounts of CO2 to be transported |
|
|
haldane effect
|
- deoxygenation promotes CO2 uptake by blood
- oxygenation promotes CO2 unloading - enhances CO2 transport by Hg - promote uptake in tissues and release in lungs - add oxygen to become more acidic - add CO2 to become more acidic |
|
|
normal blood pH
|
- temperature dependent variable
- higher the temperature = lower the pH |
|
|
fluctuation ins [H+]
|
- profound effects on body chemistry
- excitability of nerve and muscle cells - depresses CNS - increases levels cause over-excitability and muscle spams - enzyme activity - allosteric changes - renal tubules secrete H+ and K+, usually more K+ but opposite when [H+] increased - increase in [K+] alters cardiac function |
|
|
chemical buffers
|
- act as first line of defense agains changes in [H+]
|
|
|
4 buffer systems in human body
|
- H2CO3: HCO3 system = extracellular
- protein buffer system = intracellular - hemoglobin buffer system = generated from carbonic acid - phosphate buffer system = urinary buffer |
|
|
respiratory system
|
- regulates [H+] by controlling rate of CO2 removal from plasma
- adjust pulmonary ventilation - eliminates metabolically produced CO2 so the H2CO3 doesn't accumulate in body |
|
|
kidneys
|
- third and most powerful line of defense agains shifts in [H+]
- eliminate H+ - regulate HCO3- - during acidosis is secretes H+ and adds HCO3- to blood - during alkalosis it conserves H+ and eliminates HCO3- |
|
|
4 types of acid base imbalances
|
- respiratory acidosis
- respiratory alkalosis - metabolic acidosis - metabolic alkalosis |
|
|
respiratory acidosis
|
- abnormal CO2 retention
- hypo-ventilation - lung disease like COPD - anesthesia and drugs - nerve or muscle disorders that reduce efficiency of respiratory muscles - holding ones breath |
|
|
respiratory alkalosis
|
- excessive loss of CO2
- fever - aspirin poisoning that stimulate ventilation - hyperventilation |
|
|
metabolic acidosis
|
- reduction in plasma [HCO3-]
- severe diarrhea - diabetes mellitus - strenuous exercise - sever renal failure |
|
|
metabolic alkalosis
|
- vomiting
- ingestion of alkaline drugs or substances like baking soda |

