![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
gas exchange
|
- respiratory gas moves from place to place
- convection/bulk flow - simple diffusion - partial pressure determines direction - materials tend to move in net fashion from regions of high chemical potential to low chemical potential - rate proportional to difference in chemical potential |
|
|
gas laws of respiratory physiology
|
- boyle's law
- charle's law - dalton's law - henry's law |
|
|
boyle's law
|
- pressure of given quantity of gas is inversely proportional to its volume
- constant temperature |
|
|
charle's law
|
- volume of given gas quantity of gas directly proportional to its absolute temperature
- constant pressure - as temperature of gas increases, the volume occupied by gas increases |
|
|
dalton's law
|
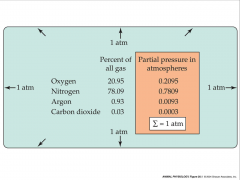
- total pressure of gas mixture is equal to sum of partial pressures of individual gases
- pressure of each gas is proportional to its concentration in mix - Px=(n/v)RT -Px is partial pressure - n is # of moles - v is volume - R is universal gas constant - T is absolute temperature |
|
|
henry's law
|
- at water-air interface
- amount of gas that dissolves in water is determined by its solubility in water and its partial pressure in air - constant temperature - solubility of a gas depends on its partial pressure above solution - partial pressure of a gas in solution is equal to partial pressure of that gas in gas phase with which solution is in equilibrium |
|
|
thoracic cavity and lung volume
|
- when thoracic cavity and lung volume expand, the pressure of air within in lungs drops below atmospheric pressure
- air flows down gradient from the outside into lungs by inhalation - exhalation is reduction of thoracic cavity and lung volume and increase of intrapulmonary pressure - aire moves down its gradient from inside lungs to outside |
|
|
partial pressure
|
- influences gas exchange between alveoli and blood
- alveolar air is humidified in nasal cavity and mixes with residual air in alveoli - alveolar air doesn't have same proportions of gases as inhaled air |
|
|
factors that affect partial pressure
|
- temperature
- solutes dissolved in liquid - water vapor - altitude |
|
|
temperature
|
- inflation of lungs is aided by warming of inhaled air in nasal cavity
- gas is cold = condenses - volume can be changed by temperature |
|
|
solutes dissolved in liquid
|
- solubility is property of solute and solvent
- rise in temperature drives dissolved gases out of solution - each grain of salt increases salinity in its vicinity, driving dissolved CO2 out of solution |
|
|
solubilities of gases in water
|
- solubilities
- temperature - presence of other solutes |
|
|
amount of gas dissolved dependent on
|
- nature of gas
- pressure - temperature - presence of solutes |
|
|
nature of gas
|
- solubility coefficient = alpha
- volume of a gas in mL at STPD that will dissolve in 1L of water when the pressure of gas is 1 atm - oxygen: 34.1 mL O2/L water - nitrogen: 16.0 mL N2/L water - carbon dioxide: 1019 mL CO2/L water |
|
|
solubility of gas
|
- more soluble the more it will dissolve into pulmonary surfactant
- higher the partial pressure of gas, the more of that gas will dissolve into pulmonary surfactant |
|
|
two factors extremely important in gas exchange
|
- relative concentration gradients of O2 and CO2 contribute to diffusion of O2 into the blood and CO2 out of blood
- concentration of CO2 isn't as great as that for O2 - solubility of CO2 in water is about 20 times that of O2 - amount of gas dissolved in fluid is determined by both its solubility in water and its partial pressure in air |
|
|
effects of pressure and temperature
|
- solubilities of gas in an aqueous solution decreases as temperature increases
- volume is inversely proportional to pressure when at constant temperature |
|
|
gas diffusion
|
- diffuse from areas of high partial pressure to areas of low partial pressure regardless of concentration
- doesn't go down concentration gradient - greater the area = greater the rate - greater the distance = slower the rate - O2 concentration is higher in cold water than warm water - partial pressure is lover in cold water than warm water - gas will diffuse from water water where [] is lower but the partial pressure is higher to cold water where [] is higher but partial pressure is lower |
|
|
Fick's law of diffusion
|
- rate of gas diffusion
- Q=KA(P1-P2)/x - Q is diffusion rate - K is diffusion constant - A is cross sectional area - X is distance separating P1 an dP2 |
|
|
diffusion coefficient
|
- contant that expresses physical conditions of the system
- temperature - molecular weight of the diffusing substance - nature of the materials through which the substances are diffusing |
|
|
Graham's law
|
- rate at which gas diffuses is inversely proportional to the square root of the molecular mass of the gas
|
|
|
O2 diffuses faster than CO2
|
- diffusion occurs faster in air than water
- met rates highest in air breathing animals - living tissue diffusion is adequate for only about 1mm from exchange site |
|
|
convective transport of gases
|
- ventilation
- bulk movement = convective flow - cellular diffusion |
|
|
flow
|
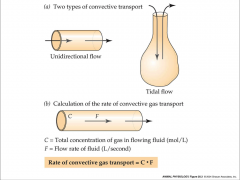
- tidal flow moves in and out the same opening
- tidal flow = most mammals - unidirectional flow goes in one way and out another - unidirectional flow = fish |
|
|
processes affect partial pressures
|
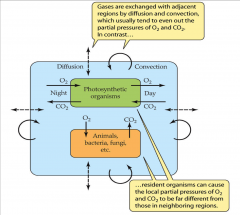
- gases are exchanged with adjacent regions by diffusion and convection
- usually tend to even out the partial pressures of O2 and CO2 - resident organisms can cause the local partial pressure of O2 and CO2 to be far different from those in neighboring regions |
|
|
alveoli
|
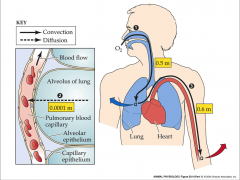
- diffusion space
- sites on the capillaries - one really thin basement membrane - short diffusion distance - O2 goes from alveoli to blood - CO2 goes from blood to alveoli |
|
|
tissue
|
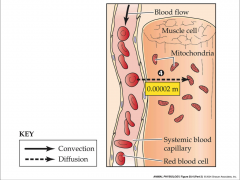
- opposite flow of O2 and CO2 from alveoli
- higher partial pressure of O2 in blood - higher partial pressure of CO2 in tissue - convection carries O2 to tissue - convection carries CO2 to lungs |
|
|
physiological oxygen cascade
|
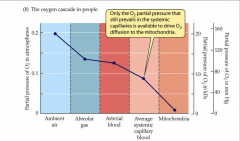
- based on analogy with cascade along mountain stream
- only O2 partial pressure that still prevails in systemic capillaries is available to dive O2 diffusion to mitochondria |

