![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are the general pKa values of the following hydrogens?
Alkyl Allylic Benzylic |
Alkyl ~ 55
Allylic - 43 Benzylic - 41 |
|
|
|
What is the pKa and general structure of:
Amine Amide |
Amine - 32-35 (:NH3)
Amide - 15-17 (Like an aldehyde, but the H is replaced with :NR2) |
|
|
|
Describe the following and how they're made:
Imine (give an example compound with answer) Enamine |
Imine is the Nitrogen analog of a carbonyl: the double-bonded Oxygen is replaced with NR3 where R can be alky, hydrogen or a lone pair.
These are also called "Schiff Bases." Example: H2N-NH2 (Hydrazine). Used in Wolff-Kishner reaction. An enamine is the Nitrogen analog of an enol, it generally rearranges to an imine (tautamerization). |
|
|
|
1) What is the Wolff-Kishner reaction and what does it do?
2) What solvent is used? Why? 3) What is the main favorable characteristic of the Wolff-Kishner reaction? |
1) It is a reaction with hydrazine (H2N-NH2) in which an aldehyde or ketone is reduced to a methylene group.
2) The solvent used is ethylene glycol because it has a high boiling point and this reaction needs to take place at high temperatures. 3) Instead of using Friedel-Crafts Alkylation (which allows for rearrangements, making the undesired product favorable), you can use Friedel-Crafts Acylation followed by Wolff-Kishner to remove (reduce) the carbonyl oxygen which avoids rearrangements in the net reaction. |
|
|
|
1) Describe the Wittig reaction.
2) What reagents are used? 3) What product does it prefer? |
1) The Wittig reaction converts carbonyls into "unambiguous" alkenes that are otherwise hard to produce. It is completely regioselective.
2) Aldehydes or Ketones plus +PPh3CH2- (Phosphorane; a ylid) 3) The (Z) or cis-product |
|
|
|
What is a ylid?
What is an example? |
1) A ylid is any compound with opposite charges on adjacent, covalently bound atoms, each of which has an electronic octet.
2) +PPh3C- (Phosphorane) |
|
|
|
1) What are the compounds LiAlH4 & NaBH4 used for?
2) How do they work? 3) Which is stronger/harsher? 4) Which is more selective? |
1) Reducing agents.
2) They donate hydride to the compound being reduced. Used for carbonyls: transforms the double-bonded oxygen to a -OH and H group attached to the same carbon. (Remember that a hydride is a hydrogen that carries a lone pair!) 3) LiAlH4 is stronger, it will reduce everything possible. (Not sure if it reduces aromatics). 4) NaBH4 is more selective, it will only reduce the most acidic group. |
|
|
|
1) What is Phosphorane?
2) How is it made? 3) Can it be made from tertiary halides? Why or why not? (Think about the structure of PPh3) |
1) A ylid (+PPh3CH2-)
2) It is made by first reacting PPh3 (triphenyl phosphine) with the desired alkyl halide (can't use tertiary, only secondary and primary) to create a phosphonim salt. Then react the phosphonium salt with butyllithium to create Phosphorane. 3) It cannot be made from tertiary halides due to steric reasons. |
|
|
|
1) Name some reducing agents.
2) What is each one used for? |
1) a)LiAlH4 (LAH); NaBH4: Used to reduce carbonyls to alcohols. LAH is harsh and completely reduces all available groups. NaBH4 is gentler and more selective.
b)LiNH3: LiNH3 selectively reduces alkynes to trans-alkynes c) Lindlar's catalyst + H2: selectively reduces alkynes to cis-alkynes d) H2 + Pd/C: completely reduces double-bonds |
|
|
|
What is the difference between NaNH2 and NaNH3(liquid) or LiNH3(liquid)?
|
NaNH2 is used to deprotonate a terminal alkyne via a free radical-like reaction. BuLi can be used, as well, but this mechanism is via the organometallic pathway.
LiNH3(liquid) and NaNH3(liquid) cause reduction of internal alkynes to trans-alkenes. Lindlar's catalyst is used to reduce internal alkynes to cis-alkenes. |
|
|
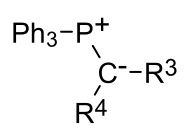
a) What is this?
b) What is it used for? |
a) A phosphorane ylid.
b) It is used in the Wittig reaction |
|
|
|
What is a hydrate?
|
A hydrate is an identical molecule with a molecule of water added. Not like a protonated alcohol, from my understanding.
Example: Ethanol (CH3–CH2–OH) can be considered as a hydrate of ethene (CH2=CH2). |
|
|
|
What is the order of precedence for naming?
REMEMBER: this is NOT necessarily the order of reactivity (need to research this, I think acid chlorides are more reactive than anhydrides and stuff) |
1) Carboxylic acid
2) Anhydride 3) Ester 4) Acid Halide 5) Amide 6) Nitrile 7) Aldehyde 8) Ketone 9) Alcohol/Phenol 10) Thiol 11) Amine 12) Large Alkyl 13) Small Alkyl |
|
|
|
Name some ortho/para directing groups.
|
1) Alkyls
2) Hydroxyl 3) Amino 4) Acylamino 5) Alkoxy 6) Phenyl 7) Halogens (special case) |
|
|
|
Name some meta directing groups.
|
1) Carboxy
2) Carboxamido 3) Carboalkoxy 4) Acyl 5) Sulfonic acid 6) Cyano 7) Nitro |
|
|
|
Halogens are what kind of directing group? Why?
|
Ortho/para, because their electron withdrawing effects are most prominent on π-bonds; they can be electron donators for π-bonds. Because they're good leaving groups, the positive charge can fall onto the carbon they're attached to, allowing them to leave the molecule.
|
|
|
|
Name some Electron Withdrawing Groups (EWGs).
|
1) Aldehyde
2) Ketone 3) Ester 4) Cyano 5) Nitro 6) Halides (special case, they're EWG to σ-bonds, but EDG to π-bonds) |
|
|
|
In reference to substituents on aromatic rings, name some Electron Donating Groups (EDGs).
|
1) Alkyl
2) Hydroxyl (OH) 3) Alkoxy (OR) 4) Amine (:NH2, :NR2, :NHR) |
|
|
|
What is the criteria for aromaticity?
|
1) 4n+2π-electrons
2) Cyclic structure 3) Overlapping p-orbitals (this usually means sp2 hybridized) 4) Conjugated π-electron system |
|
|
|
What is the criteria for anti-aromaticity?
|
1) 4n π-electrons
(there's more that I don't remember) |
|
|
|
What are two specific traits that cause a substituent to be an ortho/para director? Why?
|
1) An unshared electron lone pair on an atom directly connected to the benzene ring because the lone pair can be involved in the resonance stabilization of the carbocation intermediates.
2) An alkyl group directly attached to the benzene ring because one resonance structure will be a tertiary carbocation which stabilizes the intermediate. |
|
|
|
What are the three criteria for activating/deactivating groups?
|
1) All meta-directing groups are deactivating groups.
2) All ortho/para-directing groups (except for the Halogens) are activating groups. 3) The halogens are deactivating groups. |
|
|
|
If a benzene contains an EWG and an EDG in positions that oppose where they direct a new substituent, which group wins and why?
|
EDG because the resonance effect is stronger than the polar effect.
|
|
|
|
What governs the nucleophilicity of halide ions in polar, aprotic solvents? Why?
|
Basicity. The most basic ion is the most nucleophilic. I think this has to do with the fact that there are no hydrogens to hydrogen bond with in aprotic solvent, so the halide is free to use it's electrons as a nucleophile.
|
|
|
|
What is NaH used for?
|
It is very basic, it is a source of hydride ion. Turns alcohols into alkoxide and allows H2 gas to bubble out of the reaction mixture.
|
|
|
|
What is the difference between nucleophilicity and basicity?
|
Nucleophilicity is based on kinetics. It deals with how fast something will react. It depends on the solvent the nucleophile is in (protic solvents can decrease nucleophilicity by engaging lone pairs in h-bonding). (NEXT SIDE)
|
Basicity is thermodynamic which means it deals with the stability of the reactants vs. the products. Basicity is not dependent on the solvent it's in.
|
|
|
What is the purpose of a 3Å Molecular Sieve?
|
To absorb water from the reaction to allow it to go to completion and prevent the reaction from reversing due to hydrolysis.
|
|
|
|
What is saponification?
|
Hydrolysis of esters under basic conditions. It turns esters into carboxylic acids.
|
|
|
|
What is a desiccant?
Give an example of one. |
A desiccant is used for dehydration. P2O5 (which comes from P4O10) is a desiccant used to convert carboxylic acids into anhydrides.
|
|
|
|
1) What is PCC?
2) What is it used for? 3) What is it made of? |
1) Pyridinium chlorochromate. It is an oxidizing agent.
2) It converts primary alcohols into aldehydes and secondary alcohols into ketones. 3) Mix pyridine with HCl (hydrochloric acid) and CrO3 (chromate). |
|
|
|
What is the reactive intermediate in most addition to carboxylic acid reactions?
|
A tetrahedral addition intermediate.
|
|
|
|
Rank these seven functional groups in order of decreasing reactivity:
Acids,esters Amides Acid chlorides Nitriles Ketones Anhydrides Aldehydes |
Most
1) Acid chlorides 2) Anhydrides 3) Aldehyde ~ Ketone 4) Acids & Esters (carboxy groups) 5) Amides 6) Nitriles Least |
|
|
|
What happens when you mix an aldehyde or a ketone with a tertiary amine?
|
Nothing. Only primary and secondary amines react with aldehydes and ketones because the amine must have an alpha-hydrogen to lose to allow stabilization.
|
|
|
|
What are the important things to remember about the Wolff-Kishner reaction?
|
1) It is a condensation (loses water) followed by an elimination under basic conditions.
2) Hydrazine is used on an aldehyde or ketone (H2N-NH2) 3) BEFORE the base reacts, the Nitrogen closest to the carbonyl group loses all it's hydrogens to form an imine. |
|

