![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
128 Cards in this Set
- Front
- Back
|
alkyl halide
|
simply has halogen atom bonded to one of the sp3 hybrid carbon atom of alkyl group
|
|
|
vinyl halide
|
has a halogen atom bonded to one of the sp2 hybrid carbon atoms of an alkene
|
|
|
Aryl halide
|
has a halogen atom bonded to one of the sp2 hybrid orbital of an aromatic ring.
|
|
|
Rank the alkyl halides in the order of increasing dipole moment
|
C-I < C-Br < C-F < C-Cl
|
|
|
Alkyl Halide (haloalkane)
|
a derivative of an alkane in which one or more of the hydrogen atoms has been replaced by a halogen
|
|
|
allylic halogenation
|
substitution of a halogen for a hydrogen at the allylic position
|
|
|
allylic
|
the saturated position adjacent to a carbon to carbon double bond
|
|
|
allylic shift
|
a rearrangement that results from reaction at either end of a resonance stabilized allylic intermediate
|
|
|
anti
|
adding to (or eliminating from) opposite faces of a molecule
anti-coplanar: Having a dihedral angle of 180 Syn-coplanar: having a dihedral angle of 0 |
|
|
aprotic solvent
|
a solvent that has no acidic protons; a solvent with no O-H or N-H groups
|
|
|
aryl halide
|
an aromatic compound (benzene derivative) in which a halogen is bounded to one of the carbon atoms of the aromatic ring
|
|
|
acid/ base
|
acid donate a proton
base, an electron rich species that can abstract a proton |
|
|
concerted reaction
|
a reaction in which the breaking of bonds and the formation of new bonds occur at the same time (in one step)
|
|
|
dehydrohalogenation
|
an elimination in which the two atoms lost are a hydrogen atom and a halogen atom
|
|
|
electrophile (Lewis acid)
|
A species that can accept an electron pair from a nucleophile, forming a bond
|
|
|
Elimination
|
a reaction that involves the loss of two atoms or groups from substrate usually resulting in the forming of a pi bond
|
|
|
E1 reaction (elimination, unimolecular)
|
a mulitstep elimination where the leaving group is lost in a slow step then a proton is lost in a second step Zaitsev orientation is generally preferred
|
|
|
E2 reaction (elimination, bimolecular
|
a concerted elimination involving a transition state where the base is abstracting a proton at the same time that the leaving group is leaving the anti co planar transition state is generally preferred
|
|
|
geminal dihalide
|
a dihalide with both halogens on the same carbon atom
|
|
|
halogen exchange reaction
|
a substitution where one halogen atom replaces another commonly used to form fluorides and iodides
|
|
|
Hydride Shift
|
movement of a hydrogen atom with a pair of electrons from one atom usually carbon to another. Hydride shifts are examples of rearrangements that convert carbocations into more stable carbocation
|
|
|
inversion of configuration
|
a process in which the groups around a chiral carbon atom are changed to opposite spatial configuration usually as an result of back side attack, the SN2 reaction usually goes with this
|
|
|
leaving group
|
the atom or group of atoms that departs during substitution and elimination it can be charged or uncharged but it leaves with a pair of elections that are originally bonded to the group to the remainder of the molecule
|
|
|
methyl shift
|
rearrangement of methyl group with the pair of electrons from one atom usually carbon to another
|
|
|
nucleophile
|
a lewis base an electron rich species that can donate a pair of electrons to form a pi bond
|
|
|
nucleophilic substitution
|
a reaction where a nucleophile replace another group or atom in a molecule
|
|
|
polarizable
|
having electrons that are easily displaced toward a positive charge
|
|
|
1*, 2*, and 3* halide
|
these terms specify the substitution of the halogen bearing carbon atom called the head carbon
|
|
|
protic solvent
|
a solvent containing acidic protons usually O-H or N-H groups
|
|
|
Racemization
|
a loss of optical activity that occurs when a reation shows neither clean retention of configuration no clean inversion of configuration
|
|
|
reagent
|
the compound that serves as the attacking species in a reaction
|
|
|
rearrangement
|
a reaction involving a change in the bonding sequence within a molecule. these are common in reaction such as SN1 and E1 involving carbocation intermediates
|
|
|
retention of configuration
|
formation of product with the same configuration as the reactant in a substitution retention of configuration occurs when the nucleophile assumes the same stereochemical position in the product as the leaving group occupied
|
|
|
solvolysis
|
a nucleophilic substitution or elimination where the solvent serves as the attacking reagent solvolysis literally means cleavage by a solvent
|
|
|
stereocenter
|
an atom that gives rise to stereoisomers when its groups are interchanged the most common being asymmetric carbon atoms and a double bonded carbons in cis trans alkenes
|
|
|
stereospecific reaction
|
a reaction in which different stereoisomers react to give different stereoisomers of the product
|
|
|
substitution
|
a reaction in which an attacking species (nucleophile, electrophile, or free radical) replaces another group
|
|
|
SN2 reaction
|
substitution, nucleophilic, bi molecular) the concerted displacement of one nucleophile by another on an sp3 hybrid carbon atom
|
|
|
SN1 reaction
|
a two step interchange of nuclophiles with bond breaking preceding bond formation. the first step is ionization to form a carbocation the second step is the reaction of the carbocation with a nucleophile
|
|
|
substrate
|
the compound that is attacked by the reagent
|
|
|
vicinal dihalide
|
a dihalide with the halogens on adjacent carbon atom
|
|
|
vinyl halide
|
a derivative of an alkene in which one (or more) of the hydrogen atoms on a double bonded carbon atoms has been replaced by a halogen
|
|
|
Zaitsev's rule
|
an elimination usually gives the most substituted alkene product
|
|
|
Relative Nucleophilicity of Common Nucleophiles (in Methanol)
|
Reactivity Classification Nucleophile
Very Good Iodide(I-), Hydrogen Sulfide(HS-), Alkyl Sulfides(RS-) Good Bromide(Br-), Hydroxide(HO-), Alkoxides(RO-), Cyanide(CN-), Azide(N3-) Fair Ammonia(NH3), Chloride(Cl-), Fluoride(F-), Carboxylates(RCO2-) Weak Water(H2O), Alcohols(ROH) Very Weak Carboxylic Acids(RCO2H) |
|
|
a compound of formula CH2X2
|
this is a methylene group with two halogens and is called a methylene halide
iupac: dichloromethane |
|
|
a compound of formula CHX3
|
this is called a haloform
iupac: trichloromethane |
|
|
a compound formula CH4
|
carbon tetrahalide
iupac: tetrachloromethane |
|
|
Describe Radical Halogenation
|
it is not an effective method in the synthesis of alkyl halides because they produce a mixture of products. Bromination can be selective mostly predominatly at 3*
|
|
|
Allylic Bromination
|
bromination is highly selective, with only the most stable radical being formed.
the initiation step bromine absorbs light, causing the formation of radicals then first bromination step where bromine radical abstracts an allylic hydrogen making a allylic radical the second propagation step the allylic radical reacts with bromine |
|
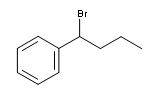
Show how free radical halogenation might be used to synthesis this compound why would you only epect a single major product
|
bromine atom will abstract the hydrogen giving the most stable radical in this case the intermediate will be stabalized by the resonance with the benzene ring
|
|
|
When discussing the SN1 mechanism explain why it is a first order reaction
|
SN1 takes place in steps unlike SN2 where the formation of the new bond and the new bond is not concerted the first step is the breaking of a bond without the formation of a new bond so it is endothermic and therefore the rate determining step the second step is fast because it is no breaking of bonds so it is highly exothermic therefore the reaction rate is only determined by the concentration of the alkyl halide
|
|
|
TOPIC: Stereochemisty
compare the possibilities when talking about SN1 and SN2 reactions and the most accurate |
3 possibilities
1)inversion of configuration 2)retention of configuration 3) Racemic mixture in a SN2 reaction inversion predominates because of back side attack this mechanism is happening concerted in a SN1 reaction the carbocation is an intermediate and is planar thus being achiral in nature and H20- can approach from any side and will result in a racemic mixture of product |
|
|
When dealing with secondary systems in substitution reaction how can you distinguish between SN2 or SN1
|
the main factor will be the strength of the nucleophile because the nucleophile is present in the SN2 rds but not in SN! the stronger the nucleophile will tend to proceed via SN2 mechanism
|
|
|
Explain the solvent effect on the SN1 and the SN2 reaction
|
in a SN2 reaction an increase in solvent polarity preferentially stabilizes the reactants thus slowing the reaction because the nucleophile is more strongly stabalized by the polar solvents than the transition state
SN1 the TS has ionic character and is more stabalized by polar solvent solvation than is the reactant so the reaction rate is increased |
|
|
The effect of the leaving group
|
the leaving group that holds that electrons best is the best therefore a weaker base will be the best because weaker bases stabilizes the negative charge better so the electron density thus making it more stable I->Br->Cl->F-
|
|
|
What is the general concept of an elimination reaction
|
Involves the loss of two atoms or groups from the substrate, usually with formation of pi bond elimination usually accompany and compete with substitutions
|
|
|
E2 Elimination reaction general mechanism
|
this can be termed by elimination in which a proton which is beta to a leaving group is removed by a base
the reaction produces alkenes. the reaction is concerted the transition state has alkene character |
|
|
The E1 mechanism
|
E1 indicated a elimination, unimolecular reaction, where rate= k[R-LG] this implies that the rate determining step depends on the decomposition of a single molecular species and this is a multi step process with the following two critical steps the loss of the leaving group to generate a carbocation intermediate and second the loss of a proton H+, from the carbocation to form the p-bond
|
|
|
In an E1 reaction what is the reactivity order of the substrate
|
(CH3)3C- >(CH3)2CH- > CH3CH2- >CH3
recall that the rate determining step is the loss of the leaving group to form a carbocation so the more stable it is the easier it is to form |
|
|
What is the effect of the leaving group in the E1 reaction
|
the only event in the rate determing step is the decomposition and breaking of the C-LG bond therefore there is a very strong dependence on the strength of the leaving group the better the leaving group the faster the reaction will take place
|
|
|
what is the effect of the Base in an E1 reaction
|
since the base is not involved in the rate determining step in an E1 reaction the nature is unimportant in the E1 reaction but the more reactive the base the more likely an E2 reaction comes
|
|
|
Explain the selectivity in an E1 reaction
|
the E1 reaction usually favors the more stable alkene as the major product more highly substituted and Trans>cis
|
|
|
The E1 reactin pathway is most common with
|
good leaving groups
stable carbocations weak bases |
|
|
Describe the E1 reaction for alkyl halides
|
step 1: cleavage of the polarized C-X bond allow the loss of the good leaving group, a halide ion, to give a carbocation intermediate this is the rate determining step (bond breaking is endothermic)
Step 2: an acid/base reaction. Deprotonation by a base from a C atom adjacent to the carbocation center leads to the creation of the Carbon carbon double bond |
|
|
Explain mechanism of the E2 reaction
|
an elimination bimolecular reaction where the
rate=k[b][R-LG] this implies that the rate determining step involvees an interaction between these two species, the base and the organic substrate this reaction is concerted so simultaneous removal of the proton H+ by the base and the loss of the leaving group LG and formation of the pi bond |
|
|
What are the effects of the R-group in an E2 reaction
|
the reaction transform 2 sp3 Carbon atoms into sp2 atoms this moves substituents further apart decreasing any steric interactions so more highly substituted systems undergo E2 eliminations more rapidly
|
|
|
wHAT Is the important thing to note when discussing elimination reactions when it comes to the base
|
the base is involved in the rate determining step in an E2 reaction so it is important so the more the reactive the base the more likely an E2 reaction will occur
|
|
|
The E2 pathway is most common
|
high concentration of a strong base poorer leaving groups
R-LG that would not lead to stable carbocations (when the E1 mechanism will occur) |
|
|
Competition between the E2 and SN2 reactions
|
since the base is also a nucleophile (the requirements for both are an unshared pair of electrons) if the base is much stronger as a nucleophile than a base like as a sulfur or halide type anions the SN2 reaction will naturally dominate
if the leaving group is attached to a primary carbon the sn2 will predominate any way because of the great rapidity of this mechanism at a primary carbon however secondary or tertuary system usually lead to predominant elimination |
|
|
E1 reaction competes with SN1
|
if the nucleophile is quite basic then elimination product predominates high temperature favors elimination
if we have a non basic but good nucleophile gives substitution product as the major product ex I- |
|
|
Topic: Physical Properties of Alkenes
Explain the trends in boiling point and density |
boiling points are close to 0 degree with densities close to 0.7 g/cm as with alkanes increase boiling points increase with molecular weights
increased branching leads to greater volatility and lower boiling points 1 |
|
|
Topic: Physical properties of alkenes
explain the polarity |
relatively non polar insoulble in water but tend to be slightly more polar than alkane for two reasons
1. the more weakly held electrons in the pi bonds are more polarizable (contributing to instantaneous dipole moments) and the vinylic bonds tend to be slightly polar ( contributing to a permanent dipole moment) alkyl groups are slightly electron donating toward a double bond helping to stabalize it thus making it have a slight postive on the alkyl group cis will have a vector sum trans will cancel out |
|
|
Topic Preperation of Alkene
Dehydration of alcohols summary |
When heated with strong acids catalysts (most commonly H2SO4, H3PO4), alcohols typically undergo a 1,2-elimination reactions to generate an alkene and water.
Also known as dehydration since it involves the removal of a molecule of water. Alcohol relative reactivity order : 3o > 2o > 1o Regioselectivity : major product is usually the more highly substituted alkene (alkene stability) Zaitsev's Rule. Stereoselectivity : trans- > cis- again controlled by stability Reaction of secondary or tertiary alcoholsusually proceeds via an E1 mechanism which proceeds via a carbocation intermediate, that can often undergo rearrangement. Primary alcohols will proceed via an E2 mechanism since the primary carbocation is highly unfavorable. Other common strong acids such as HCl, HBr or HI are less suitable catalysts as nucleophilic substitution reactions will probably interfere. |
|
|
Topic: preperation of alkene
dehydrohalogenation of alkyl halides summary |
When heated with strong bases, alkyl halides typically undergo a 1,2-elimination reactions to generate alkenes.
Typical bases are sodium or potassium hydroxides or alkoxides in the alcohol as solvent. Alkyl halide relative reactivity order : I > Br > Cl > F (nb. elimination of F is rarely used). Product distribution: Regioselectivity is usually controlled by the relative stability of the product alkenes (Zaitsev's rule). Stereoselectivity due to the preferred antiperiplanar (180o) arrangment of -H and -X in the transition state. |
|
|
alkene
|
a hydrocabon with one or more carbon to carbon double bond
diene- two double triene- three double tetraene- 4 double |
|
|
allyl group
|
a vinyl group plus a methylene group: CH2=CH-CH2
|
|
|
Bredt's rule
|
a stable bridgehead bicyclic compund cannanot have a double bond at a bridgehead position unless one of its rings contain atleast eight carbon atoms
|
|
|
dehalogenation
|
the elimination reaction of a halogen X2 from a compound
|
|
|
dehydration
|
elimination of water from a compound usually acid catalyzed
|
|
|
dehydrogenation
|
the elimination of hydrogen (H2) from a compund usually done in the presence of a catalyst
|
|
|
dehydrohalogenation
|
the elimination of a hydrogen halide (HX) from a compound usually base promoted
|
|
|
double bond isomers
|
constitutional isomers that differ only only in the position of the double bond to give the same alkane
|
|
|
element of unsaturation
|
a structural feature that results in two fewer hydrogen atoms in the molecular formula
halogens treated like hydrogen oxygen ignore nitrogencount like half a carbon |
|
|
geminal dihalide
|
a compound with two halogen atoms on the same carbon atoms
|
|
|
hofmann product
|
the least highly substituted alkane product
|
|
|
hydrogenation
|
addition of hydrogen to a molecule
|
|
|
trans-diaxial
|
an antil and co planar arrangement allowing E2 elimination of two adjacent substituents on a cyclohexane ring
|
|
|
vicinal dihalide
|
a compound with halogens on adjacent carbon atoms
|
|
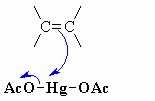
this shows step 1 of an oxymercuration-demercuration reaction explain whats going on here
|
The p electrons act as the nucleophile with the electrophilic Hg and loss of an acetate ion as a leaving group, forming the mercurinium ion.
|
|
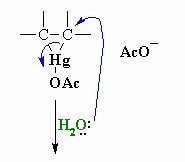
This shows step 2 of the oxymercuration- demercuration reactions what is happening
|
Water functions as a nucleophile and attacks one of the mercury substituted carbons resulting in cleavage of the C-Hg bond.
|
|
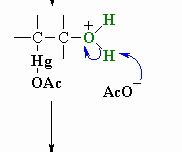
This is step 3 of Oxymercuration-demercuation reaction explain whats going on
|
The acetate ion functions as a base deprotonating the oxonium ion to give the alcohol. This completes the oxymercuration part of the reaction.
|
|

this shows the final step in the oxymercuration demercuration whats happening here
|
:(mechanism not shown)
The hydride reduces the Hg off, creating a C-H bond while breaking the C-Hg bond. This is the demercuration part of the process. |
|
|
What is a general summary for a oxymercuration - demercuration
Reaction type? Typical Reagent? Catalyst? regioselectivity? Stereoselective? |
Overall transformation C=C to H-C-C-OH
This is an alternative method for hydrating alkenes to give alcohols Typical reagents are mercury acetate, Hg(OAc)2 in aqueous THF Unfortunately, mercury compounds are generally quite toxic Regioselectivity predicted by Markovnikov's rule (most highly substituted alcohol) The reaction is not stereoselective Reaction proceeds via the formation of a cyclic mercurinium ion (compare with bromination of alkenes) The mercurinium ion is opened by the attack of water to complete the oxymercuration. When the water attacks, it does so at the more highly substituted carbon. Demercuration is effected by a reduction using sodium borohydride, NaBH4 If the reaction is carried out in the presence of an alcohol rather than water, then ethers are obtained. |
|
|
Topic: hydroboration/oxidation
write a summary for this reaction mechanism |
Reaction type: Electrophilic Addition
Summary. Overall transformation : C=C to H-C-C-OH Reagents (two steps) 1. BH3 or B2H6 then 2) NaOH/ H2O2 Regioselectivity : Anti-Markovnikov, since the B is the electrophile. Stereoselectivity : Syn since the C-B and C-H bonds form simultaneously from the BH3. The alcohol is formed over a series of steps involving the B center (see below), with retention of configuration at the C. Compliments simple hydration with opposite regiochemistry and stereospecificity |
|
|
topic: Reactions of alkene
what is the definition of addition |
a reaction involving an increase in the number of groups attached to the alkene and a decrease in the number of unsaturation
|
|
|
define: anti addition and ehat kind of reaction is this seen in
|
an addition in which two groups add to opposite faces of the double bond as in addition of Br2 which results from the bromonium ion mechanism (back side attack)
|
|
|
define: Electrophillic addition
|
an addition in which the electrophile (electron pair acceptor) bonds to one of the double bonded carbons first followed by a nucleophile
|
|
|
define: syn addition
|
an addition in which two groups add to the same face of the double bond as in Osmium tetroxide hydrooxylation
|
|
|
define: alkoxymercuration
|
the addition of mercuric acetate to an alkene in an alcohol solution, forming an alkoxymercurial intermediate. demercuration gives an ether
|
|
|
define: alpha elimination
|
the elimination of two atoms or groups from the same carbon atom freq used to form carbenes
|
|
|
define: beta elimination
|
the most common form of an elimination with two atoms or groups are removed from adjacent carbon atoms
|
|
|
define: carbene
|
a reactive intermediate with a neutral carbon atom having only two bonds and two non bonding pairs of electrons
|
|
|
define: demercuration
|
the removal of a mercury species from a molecule productof oxymercuration and alkoxymercuration usingsodium borohydride
|
|
|
epoxide (oxirane)
|
a three membered cyclic ether
|
|
|
define: halogenation
|
the addition of a halogen(x2) to a molecule or the free radical substitution of an x for an H
|
|
|
define: halohydrin
|
a beta halo-alcohol with a halogen and a hydroxyl group on adjacent carbon atoms
|
|
|
define: Halonium ion
|
a reactive, cationic intermediate with a three membered ring containing a halogen atom cl br i
|
|
|
hydration
|
the addition of water to a molecule
|
|
|
hydroboration
|
the addition of borane (BH3) or one of its derivative (BH2-THF) to a molecule
|
|
|
hydrogenation
|
the addition of hydrogen to a molecule the most common is a catalytic hydrogenation
|
|
|
hydroxylation
|
the addition of two hydroxyl groups one at each carbon of the double bond formally an oxidation
|
|
|
Markovnikov's rule
|
when a proton adds to the double bond of an alkene the proton adds to the carbon that already has more hydrogen atmos --> in an electrophilic addition to an alkene the electrophile adds in such a way as to generate the most stavle intermediate
|
|
|
oxidative cleavage
|
the cleavage of a carbon carbon bond through oxidation commonly by ozonolysis/reduction or by arm, concentrated permanganate
|
|
|
oxymercuration
|
the addition of aqueous mercuric acetate to an alkene
|
|
|
define: ozonolysis
|
the use of ozone, usually followed by reduction to cleave a double bond
|
|
|
define: peroxide effect
|
the reversal of orientation of HBr addition to alkenes in the persence of peroxides. a free radical mechanism is responsible for the peroxide effect
|
|
|
define: regioselective reaction
|
a reaction in which one direction of bond making or bond breaking occurs preferentially over all other directions addition of HCL is regioselective hydroboration-oxidation is regioselective (anti markovikov)
|
|
|
define: simmons-smith reaction
|
a cyclopropanation of an alkane using the carbenoid reagent generated from diiodomethane and the zinc copper couple
|
|
|
define: sterospecific rotation
|
a reaction that converts different stereoisomers of the starting material into different stereoisomers of the product
|
|
|
Topic: Reactions of alkenes
how many different types of Electrophilic addition reactions can occur name them |
5 that we spoke about
a. addition of hydrogen halides b.acid catalyzed hydration c. oxymercuration-demercuration d. alkoxymercuration-demercuration e. Hydroboration- oxidation |
|
|
what is the reduction reaction when discussing reactions of alkene
|
catalytic hydrogenation
|
|
|
what is the addition of carbenes most useful for
|
cyclopropanation
|
|
|
how many oxidative additions are there when discussing reactions of alkenes name them
|
there are three in which we spoke about
1. addition of halogens 2. halohydrin formation 3. syn hydroxylation |
|
|
name all the oxidative cleavage reaction
|
a. ozonolysis
b. potassium permanganate (warm) |
|
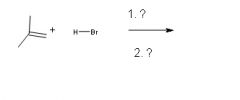
|
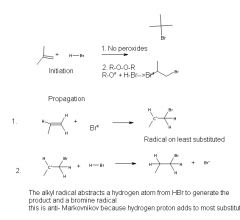
|
|
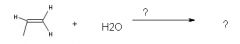
|
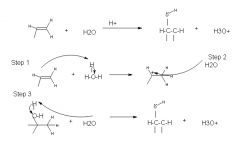
Step 1: protonation of the double bond forms a secondary carbocation
step 2: nucleophillic attack by water step 3: deprotonation gives the alcohol |
|
|
Explain the trend of increasing nucleophilicity of halide ions influenced by solvent effect
|
the key word here is polarizable
in a polar protic solvent the trend is Cl<Br<I because of the tight shell will block the hard base from reacting as a nucleophile but a soft base will not in a polar apotic solvent the trend is reversed |
|
|
Explain the steric effect of a nucleophile
|
this is the greatest effect and occur when groups attached to a nucleophillic atom are too large to allow the nucleophile to react they usually act more as bases
|

