![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
|
Octet Rule |
Atoms of different elements take part in bond formation to attain noble gas configuration |
|
|
Ionic Bond |
Complete transfer of electrons from one atom to another |
|
|
Ionization Enthalpy in Ionic Bond |
Metallic atom/Cation element to have less I.E for easy loss of electron |
|
|
Electron Gain Enthalpy in Ionic Bond |
Non-metallic atom/ anion element to have high e.g.e for easy acceptance of electron |
|
|
Lattice Energy |
Amount of energy required to separate 1 mole of ionic compd into separate oppositely charged ions |
|
|
Covalent Bond |
Bond is formed by mutual combination and sharing of electrons |
|
|
Homoatomic Molecule |
Same atoms combine. Eg- H2 |
|
|
Heteroatomic |
Different atoms make up the molecule. Eg- C2H4 |
|
|
Limitations of Octet Rule |
1. Incomplete octet of central atoms in some covalent structures. Eg- BCl3 2. Some compds have odd no of total electrons. Eg- NO2 3. Expanded octet of 3rd period elements 4. Noble gases make compounds |
|
|
Bond Length |
Equilibrium distance between the centres of the nuclei of the two bonded atoms. |
|
|
Bond Angle |
Angle b/w lines representing the orbitals containing the bonding |
|
|
Bond Enthalpy |
Amount of energy required to break one mole of bonds of a particular type to separate them into gaseous atoms. |
|
|
Bond Order |
No of bonds b/w a covalent structure. Eg- H2 has b.o=1 (H-H) |
|
|
Non-Polar Covalent Bond |
When the atoms joined by covalent bonds are the same like H2, Cl2, etc the shared pair of electrons is equally attracted and is equidistant from each. |
|
|
Polar Covalent Bond |
When two dissimilar atoms make a covalent bond like HCl the more electronegative atom will pull the electron pair towards it so both atoms develop partial charge. |
|
|
Dipole Moment |
Product of magnitude of charge of + and - charge and the difference between them. µ = Q x d |
|
|
VSEPR |
Valence Shell Electron Pair Repulsion Theory |
|
|
Postulates of VSEPR |
1.shape depends on no of e- pairs 2.e- pairs repel each other 3.e- pairs try to be in a position of min repulsion 4.valence shell is taken as a sphere w the electron pairs placed at min distance. 5.multiple bond treated as a single e- pair |
|
|
VBT |
Valence Bond Theory |
|
|
Postulates of VBT |
1.bond formed from overlapping of orbitals 2.overlapping orbital contain a pair of e- 3.e- density concentrates b/w bonded atoms 4.strength of the bond depends on degree of overlap |
|
|
Sigma Bonds |
involves s orbitals, strong bond, axial overlap |
|
|
Pi Bond |
does not include s orbitals, comparatively weak, sidewise overlap |
|
|
Format of VSEPR |

1.central atom 2.valence electron of c.a 3.no of b.p and l.p 4.total e- pairs 5.geometry 6.shape |
|
|
BeCl2 |
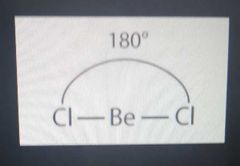
Linear, 180 sp |
|
|
BF3 |
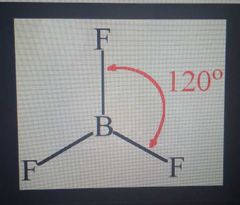
Trigonal Planar, 120 sp2 |
|
|
CH4 |
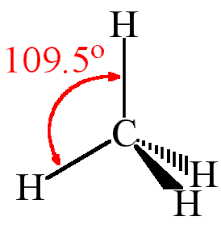
Tetrahedral, 109 28' sp3 |
|
|
PCl5 |
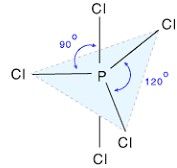
Trigonal Bipyramidal ,90 and 120 sp3d |
|
|
SF6 |
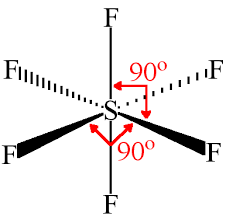
Octahedral, 90 sp3d2 |
|
|
IF7 |
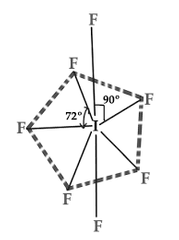
Pentagonal Bipyramidal sp3d3 |
|
|
AB2 type molecule |
B.P-2 L.P-1 Geometry- trigonal planar Shape-Bent eg- SO2 |
|
|
AB3 type molecule |
B.P- 3
L.P-1 Geometry- tetrahedral Shape- pyramidal eg- NH3 |
|
|
AB4 type molecule |
B.P- 4
L.P-1 Geometry- trigonal bypyramidal Shape- see-saw eg- SF4 |
|
|
AB2 with 2 L.P |
B.P-2 L.P-2 Geometry-tetrahedral Shape- bent eg-H2O |
|
|
AB5 type molecule |
B.P- 5 L.P-1 Geometry- octahedral Shape- square pyramid eg- BrF5 |
|
|
AB3 with 2 L.P |
B.P- 3 L.P-2 Geometry- TBP Shape- T-shaped eg- ClF3 |
|
|
AB2 with 3 L.P |
B.P- 2 L.P-3 Geometry- TBP Shape- linear eg- XeF2 |
|
|
AB4 with 2 L.P |
B.P-4 L.P-2 Geometry- octahedral Shape-square planar eg- XeF4 |
|
|
AB6 type molecule |
B.P- 6
L.P-1 Geometry- pentagonal bipyramidal Shape-distorted octahedron eg- XeF6 |
|
|
Bond Angle Comparison |
Depends on electronegativity of central atom. Higher the electronegativity higher the bond angle. Repulsion b/w surrounding atoms should be less for higher bond angle |
|
|
Hybridisation |
homogenisation of orbitals of valence shell w/ diff. energy & orientation to give new set of orbitals w/ similar shape and energy |
|
|
Resonance |
phenomenon of existence of two or more possible lewis dot structures of a molecule or ion |
|
|
Resonatng Structures |
diff structures for the same molecule/ion |
|
|
Molecular Orbital Theory |
1.e- of a molecule are present in various mol orbitals 2.e- are influenced by two or more nuclei 3.two atomic orbs combine to form 1 bonding orb and 1 antibonding orb |
|
|
Hydrogen Bond |
dipole-dipole interaction b/w between H of a molecule/ion carrying + charge and - charge on F,O,N,etc |
|
|
Dipole |
system of 2 equal and opposite charges separated by a small distance |
|
|
Dipole Moment |
Dip. Mom. (μ)= charge(Q) x distance(d) vector quantity , SI unit- Debye (D) 1D= 3.33 x 10^-30 Cm |
|
|
LCAO |
Linear Combination of Atomic Orbitals |
|
|
Backbonding |
delocalisation of charge b/w 2 atoms involved in a sigma bond and one of them has a filled orbital having one l.p and the other has a vacant orbital. |
|
|
Fajan's Rule |
when a cation having high polarising power approaches an anion having high polarizability then the bond b/w them is predominantly covalent |
|
|
Polarizability |
measure of tendency to allow distortion of its e- cloud |

