![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
84 Cards in this Set
- Front
- Back
|
What systemic diseases are associated with Nephrotic Syndrome?
|
- Diabetic Nephropathy
- Amyloidosis - Light Chain Deposition Disease |
|
|
What are the hereditary glomerular diseases?
|
- Alport syndrome
- Thin basement membrane disease |
|
|
What is the leading cause of ESRD in most western societies?
|
Diabetic Nephropathy (related to DM type I and II)
30-40% of patients will develop nephropathy |
|
|
How does Diabetes Mellitus relate to Nephropathy?
|
- Both DM type 1 and 2
- Risk is related to duration of disease |
|
|
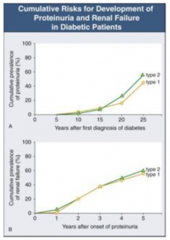
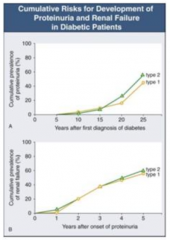
How long does it take after first diagnosis of diabetes to get proteinuria?
|
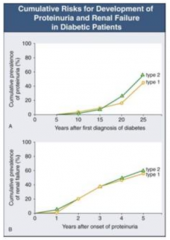
- Takes 5-10 years until some people start getting proteinuria
- At 25 years after diagnosis, ~50% have proteinuria |
|
|
How long after onset of proteinuria with DM does it take to get renal failure?
|
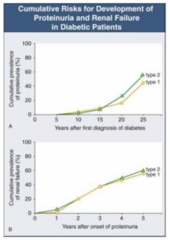
- Once proteinuria begins, you can get renal failure at any time
- 5 years after onset, ~50% have renal failure |
|
|
When should a person with DM be worried about renal failure?
|
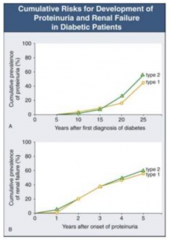
Once they start getting proteinuria
|
|
|
How is the nephron affected by DM?
|
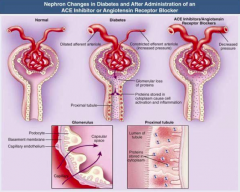
- Dilated afferent arteriole (glucose-dependent, via vasoactive mediators VEGF, NO, TGF-B)
- Constricted efferent arteriole (increased pressure) - Glomerular loss of proteins - Proteins stored in cytoplasm cause cell activation and inflammation |
|
|
What are the implications of a dilated afferent arteriole and a constricted efferent arteriole in DM? Is this fixable?
|
- Causes hyperfiltration
- Increases colloid osmotic pressure in post-glomerular capillaries - Increases Na+ reabsorption in PT - Can be corrected w/ good glycemic control |
|
|
How does angiotensin II affect nephron?
|
Causes hypertrophic PT growth
|
|
|
What are the changes to kidney function in diabetic nephropathy?
|
- Hyperfiltration
- Hypertrophy of kidney (may increase by several cm) - Mesangial changes (increase in number and size) - Proteinuria - Fibrosis |
|
|
What is hypertrophy of the kidney in DM/diabetic nephropathy associated with?
|
Increase in number of mesangial cells and capillary loops (increases filtration surface area)
|
|
|
How does the mesangium change in diabetic nephropathy? What mediates this?
|
- Mesangial expansion (increase in cell number and size)
- Nodular diabetic glomerulosclerosis (increased deposition of extracellular matrix) - Mediated by both glucose and glucose-derived AGEs (Advanced glycation end products) |
|
|
What causes proteinuria in Diabetic Nephropathy?
|
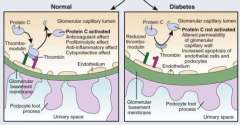
- Widening of GBM (accumulation of type IV collagen and net reduction in negatively charged heparin sulfate)
- Podocyte changes (increased width of foot processes, apoptosis, reduced migration preventing coverage of BM) * Serum proteins cross the BM d/t disrupted texture, gaps, and holes * |
|
|
What causes apoptosis of podocytes in diabetic nephropathy? Implications?
|
- Triggered by AngII and TGF-B
- Leads to changes in podocytes that causes proteinuria |
|
|
What causes reduced migration of podocytes in diabetic nephropathy? Implications?
|
- Reduced by AngII
- Prevents coverage of BM by podocytes, leading to proteinuria |
|

What is normal / abnormal?
|

- Left: normal BM and podocyte
- Right: BM and podocyte changes associated with diabetic nephropathy |
|
|
What kind of fibrosis is seen in Diabetic Nephropathy? Cause? Implications?
|
- Tubulointerstitial fibrosis (correlates w/ prognosis)
- Caused by release of TGF-β and AngII - Tubular cells change their phenotype and become fibroblasts - High glucose conc. and AGEs (Advanced glycation end products) further stimulate this process |
|
|
What are the stages of Diabetic Nephropathy?
|
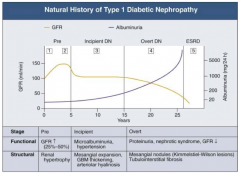
- Pre (1 and 2)
- Incipient (3) - Overt (4) - ESRD (5) |
|
|
What happens to GFR across the stages of Diabetic Nephropathy?
|
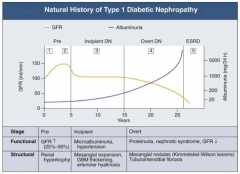
- Pre (1 and 2): GFR ↑ (25-50%)
- Incipient (3): returns to normal level - Overt (4): GFR ↓ - ESRD (5): very low GFR |
|
|
What happens to albuminuria across the stages of Diabetic Nephropathy?
|
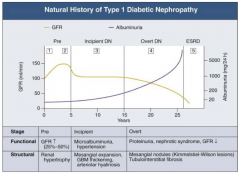
- Pre (1 and 2): little change
- Incipient (3): small increase - Overt (4): greater climb - ESRD (5): very high |
|
|
What are the functional and structural characteristics of Pre (Stage 1) Diabetic Nephropathy?
|
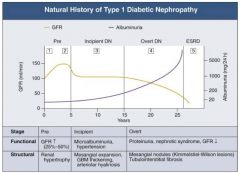
Onset of Diabetes:
- GFR ↑ d/t glomerular hyper-filtration - Glomerular hypertrophy seen on biposy - Renal size ↑ - Reversible, transient albuminuria |
|
|
What are the functional and structural characteristics of Pre (Stage 2) Diabetic Nephropathy?
|
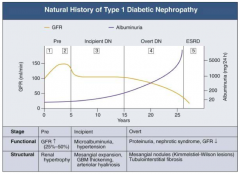
Clinically Asymptomatic, but Biopsy shows:
- Mesangial expansion - GBM thickening |
|
|
What are the functional and structural characteristics of Incipient (Stage 3) Diabetic Nephropathy?
|
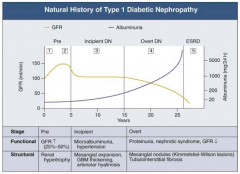
Early Nephropathy
- Development of HTN - Persistent micro-albuminuria by 24-hr collection - Urinary albumin excretion 30-300 mg/day |
|
|
What are the functional and structural characteristics of Overt (Stage 4) Diabetic Nephropathy?
|
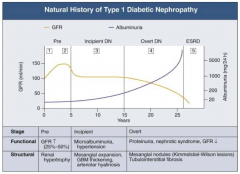
Overt Proteinuria:
- Urinary albumin > 300 mg/day - GFR starts to decline - 50% of patients will reach ESRD within 7-10 years - Retinopathy presents in 90-95% patients |
|
|
What are the functional and structural characteristics of ESRD (Stage 5) Diabetic Nephropathy?
|
End-Stage Renal Disease:
- Renal replacement therapy necessary - Occurs a mean of 15 years after onset of Type 1 DM in patients who develop proteinuria (30%) |
|
|
What are Kimmelstiel-Wilson lesions?
|
- Acellular
- Nodular diabetic glomerulosclerosis |
|
|
What are the co-morbidities of DM?
|
- HTN
- Neuropathy - Vascular changes - Increased mortality |
|
|
What features of DM lead to HTN?
|
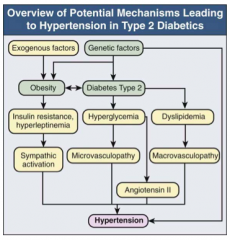
- Obesity
- Sympathetic activation d/t insulin resistance and hyperleptinemia - Microvsculopathy d/t hyperglycemia - AngII d/t hyperglycemia, macrovasculopathy, and dyslipidemia |
|
|
How common is Diabetic Retinopathy?
|
- Almost all patients w/ Type 1 diabetes w/ nephropathy
- In 50-60% of Type 2 diabetes w/ nephropathy |
|
|
What are the characteristics / syptoms of neuropathy in diabetic nephropathy?
|
- Sensory polyneuropathy: diabetic foot
- Autonomic polyneuropathy: silent angina, gastroparesis, erectile impotence, detrusor paresis |
|
|
What are the macrovascular complications seen in diabetic nephropathy?
|
- Stroke
- Coronary heart disease - Peripheral vascular disease |
|
|
How does microalbuminuria and macroalbuminuria affect diabetic nephropathy mortality?
|
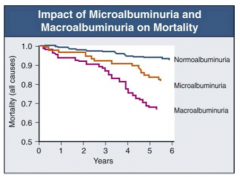
Macroalbuminuria >> Microalbuminuria >> Normoalbuminuria
|
|
|
How do you treat Diabetic Nephropathy?
|
- HTN therapy
- Glucose control - Reduction of proteinuria - Lipid lowering therapy - Life style modification |
|
|
How does HTN relate to DN?
|
- In DM patients w/ DN, HTN is almost always present
- Uncontrolled HTN associated with more rapid progression of DN and increased risk of fatal and nonfatal CV events |
|
|
What is the goal BP for diabetics? How do anti-HTN therapies affect survival?
|
- Goal: 130/80 mmHg (or lower)
- Anti-HTN therapies improve survival in both type 1 and 2 DM w/ DN |
|
|
How does glucose control affect DN?
|
- Good glycemic control (HbA1c < 7%) decreases risk of DM type 1 developing ESRD after 25 years from 40% to 9%
- Reduces progression to microalbuminuria - Decreases microvascular complications (ie retinopathy) - Decreases CV sequelae (even after later deterioration in glycemic control) |
|
|
How do you reduce proteinuria?
|
Renin-angiotensin-aldosterone system blockade
|
|
|
How does renin-angiotensin-aldosterone system blockade affect DN?
|
- Reduces proteinuria
- Renoprotective independent of BP - May cause up to 30% decline in GFR, but reno-protective in long-term - Works through renal hemodynamic changes and blocking non-hemodynamic effects of AngII |
|
|
What are the typical lipid levels in DN?
|
- Low HDL
- High TGs - Smaller LDL particles |
|
|
How are lipids controlled in DN? Guidelines?
|
- In type 2 DM w/ DN, tx w/ statins provides substantial CV benefit
- Goal: LDL <100 mg/dl in general and <70 mg/dl w/ CVD |
|
|
What lifestyle modifications should patients w/ DN do? Implications?
|
- Smoking cessation - decreases progression of micro to macro albuminuria
- Weight reduction - possibly improves renal outcome via reduction in proteinuria |
|
|
In Type 1 DM, how should you manage a patient with normoalbuminuria / normotension?
|
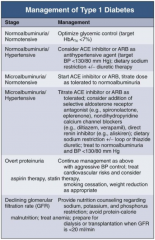
Optimize glycemic control (target HbA1c < 7%)
|
|
|
In Type 1 DM, how should you manage a patient with normoalbuminuria and hypertension?
|
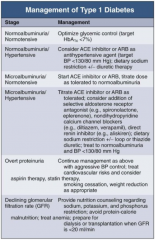
- Consider ACE-I or ARB as anti-HTN agent (target BP < 130/80 mmHg)
- Dietary Na+ restriction +/- diuretic therapy |
|
|
In Type 1 DM, how should you manage a patient with microalbuminuria and normotension?
|
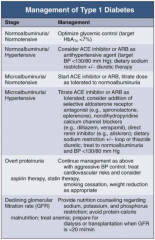
- Start ACE-I or ARB
- Titrate dose as tolerate to normoalbuminuria |
|
|
In Type 1 DM, how should you manage a patient with microalbuminuria and hypertension?
|
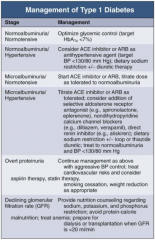
- Titrate ACE-I or ARB as tolerated
- Consider addition of: -- Selective aldosterone receptor antagonist (eg, spironolactone, eplerenone) -- Non-dihydropyridine CCB (eg, diltiazem or verapamil) -- Direct renin inhibitor (eg, aliskiren) - Dietary Na+ restriction +/- loop or thiazide diuretic - Treat to normoalbuminuria and BP < 130/80 mmHg |
|
|
In Type 1 DM, how should you manage a patient with overt proteinuria?
|
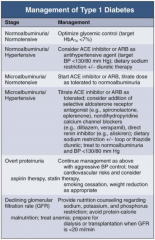
- Continue management as for microalbuminuria and hypertension w/ aggressive BP control
- Treat CV risks and consider aspirin therapy and statin therapy - Lifestyle modifications: smoking cessation and weight reduction as appropriate |
|
|
In Type 1 DM, how should you manage a patient with declining GFR?
|
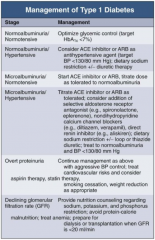
- Provide nutrition counseling regarding Na+, K+, and phosphorus restriction
- Avoid protein-calorie malnutrition - Treat anemia - Prepare for dialysis or transplantation when GFR <20ml/min |
|
|
What are the non-diabetic nephrotic syndromes?
|
- Amyloidosis
- Light chain deposition disease |
|
|
What is Amyloidosis?
|
- Generic term for a family of diseases defined by morphologic criteria
- Characterized by deposition in extracellular spaces of a proteinaceous material |
|
|
What kind of proteins cause amyloidosis in the kidney?
|
- Light chains (secreted by a single clone of B cells)
- Lambda light chains (AL) |
|
|
What are light chains released from? What is it associated with?
|
- Secreted by a single clone of B cells
- 20% of cases associated with multiple myeloma - Type of amyloidosis affecting the kidney |
|
|
What causes systemic amyloidosis?
|
Chronic inflammation
|
|
|
What are the kidney manifestations of amyloidosis?
|
- Enlarged
- Proteinuria, mainly albuminuria - Absence of microscopic hematuria - Tubular defects from amyloid deposits - Renal tubular acidosis (Fanconi syndrome) - Polyuria - polydipsia |
|
|
What syndrome is renal tubular acidosis a part of?
|
Fanconi syndrome
|
|
|
How can you diagnose Amyloidosis?
|
- LM: deposits
- Congo-red stain - apple green birefringence - IM: staining for light chain |
|
|
Which organs can be affected by amyloidosis?
|
May infiltrate any organ other than the brain
|
|
|
What are the extra-renal manifestations of amyloidosis?
|
- Restrictive CM (1/3)
- GI: motility disturbances, malabsorption, hemorrhage, or obstruction - Macroglossia (large tongue) - Splenomegaly - Peripheral nerve: sensory polyneuropathy, autonomic neuropathy (orthostatic HTN), lack of sweating, bladder dysfunction, impotence - Skin: purpura (around eyes), papules, nodules, and plaques, occurring usually on face and upper trunk - Joint: shoulder pain and swelling |
|
|
What component of immunoglobulin can be deposited in the kidney?
|
Usually κ light chain
|
|
|
What is light chain deposition disease associated with?
|
50% of cases coexist w/ multiple myeloma
|
|
|
What symptoms do patients with light chain deposition disease develop?
|
- Proteinuria
- Hematuria - Chronic renal insufficiency |
|
|
How can you diagnose light chain deposition disease develop?
|
- LM: nodular glomerulosclerosis
- IF: light chain staining (κ) - EM: granular deposits along GBM |
|
|
How can you distinguish the types of amyloidosis?
|
- Biopsy of superficial organ / kidney specimen
- Congo-Red Stain: → +: amyloidosis (lambda light chains usually) → -: stain w/ anti-κ/λ (light chain deposition disease) |
|
|
What are the types of hereditary glomerular disease?
|
- Alport syndrome
- Thin Basement Membrane |
|
|
How is Alport syndrome inherited?
|
- 80% X-linked recessive
- Can be autosomal recessive too |
|
|
What mutation causes Alport Syndrome? What does it encode? Implications?
|
- COL4A5 gene on chromosome Xq22
- Encodes α5 chain of type IV collagen - Leads to defect in basement membrane |
|
|
What are the renal symptoms of Alport Syndrome?
|
- Hematuria
- Proteinuria - HTN - ESRD in all affected males w/ X-linked AS (90% by age 40) |
|
|
What are the characteristics of hematuria in Alport Syndrome?
|
- Males have persistent microscopic hematuria
- Episodic gross hematuria, precipitated by URI - First 2 decades of life - More than 90% of females w/ X-linked AS have persistent or intermittent microscpic hematuria (but 7% of obligate heterozygotes never manifest hematuria) |
|
|
What are the characteristics of proteinuria in Alport Syndrome?
|
- Absent early
- Develops eventually in all males w/ X-linked AS and females w/ Autosomal-Recessive AS |
|
|
What determines the rate of development of ESRD in Alport Syndrome?
|
- Rate determined by underlying COL4A5 mutation
- 12% females w/ X-linked AS develop ESRD before age 40, 30% by 60, 40% by 80 - 90% of males w/ X-linked AS develop ESRD by age 40 |
|
|
Is Alport Syndrome more severe in males or females?
|
Males
- 12% females w/ X-linked AS develop ESRD before age 40, 30% by 60, 40% by 80 - 90% of males w/ X-linked AS develop ESRD by age 40 |
|
|
What are the extra-renal manifestations of Alport Syndrome?
|
- Cochlear defects
- Ocular defects - Leiomyomatosis |
|
|
What are the characteristics of cochlear defects in Alport Syndrome? Who is affected more by it?
|
- Adherence defect of organ of Corti to basilar membrane
- 80% of males - 20-30% of females |
|
|
What are the characteristics of ocular defects in Alport Syndrome? Who is affected more by it?
|
- Anterior lenticonus, pathognomic
- Maculopathy, whitish or yellowish flecks or granulations in a perimacular distribution - 30-40% of XLAS males - 15% of XLAS females |
|
|
What are the characteristics of leiomyomatosis in Alport Syndrome? Who is affected more by it?
|
Esophagus and tracheobronchial tree
|
|
|
How do you diagnose Alport Syndrome?
|
- LM: early in disease glomeruli may appear normal; later global and segmental glomerulosclerosis, interstitial fibrosis
- IF: negative or non-specific IgM, C - EM: variable thickening, thinning, basket weaving, and lamellation of GBM |
|
|
How do you treat Alport Syndrome?
|
- No-disease specific therapy (RAAS blockade)
- Renal replacement is eventually necessary - Transplant: 2-3% will get anti-GBM disease |
|
|
What is the other name for thin basement membrane disease?
|
Benign Familial Hematuria
|
|
|
How is thin basement membrane disease (benign familial hematuria) inherited?
|
Usually autosomal dominant inheritance
|
|
|
What are the features of thin basement membrane disease (benign familial hematuria)?
|
- Continuous or intermitten microhematuria
- With or without gross hematuria - Generally no renal insufficiency - Previously considered benign (proteinuria, HTN, and ESRD are unusual) - Extra-renal features are rare |
|
|
How do you diagnose thin basement membrane disease (benign familial hematuria)?
|
** EM: thin GBM (usually ≤ 200 nm)
- LM: normal glomeruli - IF: negative |
|
|
How do you treat thin basement membrane disease (benign familial hematuria)?
|
- Reassurance
- Should be followed: BMP, urinalysis, and BP monitored every 1-2 years |
|
|
Does thin basement membrane disease (benign familial hematuria) progress to ESRD?
|
Very small, but real, risk of progression to ESRD
|
|
|
What is the most common cause of end-stage renal disease in US?
|
Diabetes Mellitus
|

