![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
64 Cards in this Set
- Front
- Back
|
3 ways to form a Markovnikov alcohol |
Nucleophilic substitution, ex. NaOH
Hydration: H3O+
1) Hg(OAc), H2O 2) NaBH4 |
|
|
How to form an anti-Markovinov alcohol |
1) BH3 * THF 2) H2O2, NaOH |
|
|
How to form an anti diol |
1) MBPCA 2) H3O+ |
|
|
2 ways to form a syn diol |
OsO4 H2O2
or
Cold KMnO4 -OH |
|
|
What is the hybridization of oxygen in water and methanol? What is the shape of these structures? |
sp3 Tetrahedral |
|
|
What is a hydroxyl? |
When an OH group is a substituent instead of on the parent chain |
|
|
Order of naming priority of functional groups 1-5 AND their suffixes
What happens if more than one functional group is present? |
1) Acids -anoic acid 2) Esters -oate 3) Aldehydes -al 4) Ketones -one 5) Alcohols -ol
The lower priority group is treated as a substituent |
|
|
Why do alcohols have higher boiling points? |
Hydrogen bonding between molecules |
|
|
How is solubility in water affected by the size of the alkyl group in a molecule?
Why? |
As the alkyl's size increases, solubility decreases
The alkyl group is hydrophobic and disrupts the hydrogen bonding of the hydroxy and water molecules |
|
|
How does the cyclic shape of a molecule affect its solubility?
Why? |
Increases solubility because it reduces steric hinderance |
|
|
Are alcohols soluble in nonpolar solvents?
Why? |
Yes. B/c the alkyl is soluble in this environment |
|
|
What is essentially happening when an acid disassociates? |
The acid molecule rips away the electrons from the acid hydrogen, and releases the hydrogen |
|
|
What 4 things affect acidity of a molecule? |
1) Halogens and other electron withdrawing (electroneg) groups INCREASE acidity, and vice versa; hence: 2) The MORE carbons in a molecule, the LOWER the acidity: Bigger = lesser 3) Proximity of these groups to the acid hydrogen 4) Resonance stabilization LOWERS acidity |
|
|
What is the relationship between pka and acidity? |
Inverse |
|
|
If you have a strong nucleophile, what will occur under primary, secondary, and tertiary conditions?
If you have a weak nucleophile? |
Strong Primary = Sn2 Secondary = Sn2 Tertiary = E2
Weak Primary = no rxn Secondary = Sn1/E1 Tertiary = Sn1/E1 |
|
|
How are alkoxide ions formed?
What 3 reagents are used for 1, 2, 3 alcohols? |
Primary = Na Secondary or tertiary = K OR NaH/THF for all three
ROH + NaH/THF -> RO- +Na |
|
|
Why will an organometallic reagent attack carbon? |
- When C is bonded to a metal (Mg or Li) it has a partial positive charge - Carbon acts as a nucleophile and attacks a partially positive carbon (bonded to an electron withdrawing group) |
|
|
How is a Grignard reagent formed? Include ALL reagents for this rxn |
R-X + Mg ----ether----> R-Mg-X
Ether necessary as solvent to stabilize the complex |
|
|
How is an organolithium reagent formed? |
R-X + 2Li ----> R-Li + Li-X
Ether as solvent not necessary |
|
|
What is different about a Grignard reaction with acid chlorides and esters, than a regular carbonyl?
What happens? 4 steps
Draw it! |
This reaction needs 2 moles (eq.) of Grignard reagent!!
First mole produces a tetrahedral intermediate, breaking the double bond and giving oxygen a negative charge ---> UNSTABLE
O's negative charge resonates into a double bond to reform a carbonyl ---> EJECTS the chlorine or -OR or ester to form a ketone intermediate!!!
Second mole of Grignard attacks carbonyl, forming a tertiary alkoxide
Protonation forms alcohol |
|
|
2 limitations for Grignard and organolithium reagents |
1) Must be in an aprotic environment, otherwise will act as a strong base and be deactivated by taking an acidic H 2) No other electrophilic multiple bonds can be present to act as competition to the carbonyl |
|
|
What are 4 groups to look out for which could compromise a Grignard/organolithium rxn? |
Protic compounds with acidic protons
1) O-H 2) N-H 3) S-H 4) Terminal alkynes |
|
|
What is a thiol? AKA?
How are they names? |
A sulfer analogue of alcohol AKA mercapto group -SH
Named by adding -thiol to the alkane name, but don't drop the -e suffix
Any alcohol rxn would occur the same way with a thiol group |
|
|
How are thiols synthesized?
How to prevent dialkylation (R-S-R)? |
Thiolate ion (HS-) attacks a alkyl halide in an Sn2 mechanism (consider type of carbon in a problem)
Product: R-S-H
To prevent dialkylation, use a large excess of of thiolate ion to the alkyl halide |
|
|
What essentially is a reduction reaction? |
Adding hydrogens and removing oxygen bonds or oxygens |
|
|
What is the order of most reduced alcohol to most oxidized? |
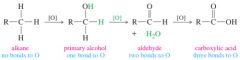
|
|
|
What are the 2 reducing agents of carbonyl compounds? Which is stronger? What will each reaction with?
Why do these two reagents work? |
1) NaBH4 -> weaker Reacts with aldehydes and ketones ONLY
2) LiAlH4 -> stronger Reacts with esters and carboxylic acids + aldehydes and ketones
These 2 reagents are sources of hydrides (H-), which acts as nucleophiles for an Sn2 mechanism |
|
|
What is the 2 step mechanism of an NaBH4 reduction?
Draw it! |
1) The hydride attacks the carbonyl carbon to break the double bond and push the electrons resonate up to the oxygen, giving it a negative charge 3) Protonation creates the alcohol |
|
|
What is the step mechanism of an LiAlH4 reduction?
What are the products??
Draw it! |
1) FIRST hydride attacks the carbonyl carbon to break the double bond and push the electrons resonate up to the oxygen, giving it a negative charge (same as NaBH4) 2) Oxygen's extra electron pair resonates to reform the double bond and push out the ester or alcohol group 3) SECOND hydride attacks the carbonyl again, same way as first step 4) Protonation creates alcohol
Products are 2 alcohols in the case of an ester, or an alcohol and water in the case of a carboxylic acid |
|
|
What essentially is an oxidation reaction?
What is the pattern? |
Adding oxygen bonds or oxygens and removing hydrogens
Add an oxygen as OH, add a double bond to that oxygen (carbonyl), add an oxygen as OH |
|
|
What are the 2 oxidizing agents? Which is stronger? |
1) PCC -> weaker
2) Chromic acid -> stronger |
|
|
Which 3 compounds cannot be any further oxidized? |
1) Carboxylic acid 2) Ketone 3) Tertiary alcohol |
|
|
What is the product from oxidation of a primary, secondary and tertiary alcohol? |
1° alcohol --> aldehyde --> carboxylic acid 2° alcohol --> ketone (no matter the reagent) 3° alcohol --> no rxn |
|
|
When you oxidize a primary alcohol, what must be considered? |
The strength of the oxidizing agent: - PCC will take it only one step to an aldehyde - Chromic acid is too strong to stop at an aldehyde, it will go straight to carboxylic acid |
|
|
Why would we want to convert an alcohol into a tosylate?
How is this done? |
Turns the OH into a good leaving group for an Sn2 on primary and secondary alkyls
R-OH + TsCl/pyridine ---> R-OTs |
|
|
What is the product when R-OTs reacts with NH3? |
NH3 is a strong nucleophile
R-OTs + NH3 --> R-NH2 |
|
|
What happens when R-OH reacts with NH3? |
No reaction b/c NH3 is not a strong enough base to abstract a proton from the alcohol |
|
|
What are the 2 methods of reducing an alcohol? What are the reagents? What is the product of this? |
1) Dehydration elimination with H2SO4 --> alkene + H2/Pt ---> alkane
2) Make a tosylate, then reduce it with LiAlH4 R-OH + TsCl/pyridine ---> R-OTs + LiAlH4 ---> alkane |
|
|
What is a hydohalic acid?
What happens when alcohols react with hydrophilic acids? |
Hydrohalic acids = HX
1) Alcohol is protonated by HX, turning it into a good leaving group and leaving behind X- 2) X- is a weak base (anion of a strong acid) but are strong nucleophiles --> Sn1 or Sn2 depending on structure of alcohol |
|
|
What does HCl need that HBr does not in order for a substitution reaction to occur with an alcohol?
Why? |
HCl needs ZnCl2 to promote the reaction b/c Cl- is a weaker nucleophile than Br --> Br- can work alone |
|
|
If I want to create an alkyl chloride, bromide, or iodide from an alcohol, what are the best reagents to use and when? |
Primary = SOCl2, PBr3, P/I2 Secondary = same as primary Tertiary = HCl, HBr, HI |
|
|
What is the 2 step mechanism for a PBr3 reaction?
What effect does this have on configuration?
Draw it! |
1) Oxygen of alcohol attacks the phosphorous, kicking out one of the bromines -> O-P-Br complex is an excellent leaving group 2) Br- ion Sn2 backside attacks the alcohol carbon, Sn2 ejecting the O-P-Br complex --> yields R-Br
Inversion of configuration! |
|
|
What does the mechanism of an HCl and SOCl2 substitution resemble?
What effect does this have on configuration? |
Both resemble Sn1, despite that SOCl2 is used for primary and secondary alkanes |
|
|
What is necessary for a pinacol rearrangement to occur? What is the mechanism? |
Molecule must be a vicinal diol
1) Acid protons the HIGHEST degree hydroxyl group 2) Water leaves, leaving behind a positively charged carbon 3) A methyl shift forms a resonance stabilized carbocation between the remaining OH and its now positive carbon (draw resonance) 4) Water deprotonates the OH and leaves behind a ketone |
|
|
Where does the ketone always end up in the product of a pinacol rearrangement? |
On the lower degree hydroxyl group |
|
|
Why do ethers make good solvents? |
They are relatively unreactive |
|
|
How to do common naming for ethers? |
Name the two alkyl groups attached to the oxygen in alphabetical order and add the word "ether"
If ether is symmetrical, use the prefix "di-" |
|
|
When do you use the IUPAC naming of ethers, and how is it done? |
When one end of the ether is very complex
- The more complex alkyl group is the alkane parent chain - The other end now includes the oxygen and is a substituent called "alkoxy" - substitute the "alk" portion for whatever prefix denotes the size of the alkane in it |
|
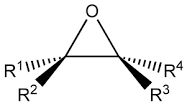
What are the TWO common names for this ether? |
Epoxide
Oxirane |
|
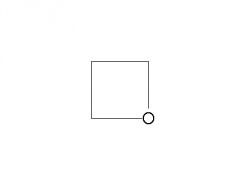
What is the common name for this ether? |
Oxetane |
|
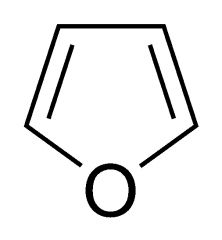
What is the common name for this ether? |
Furan |
|
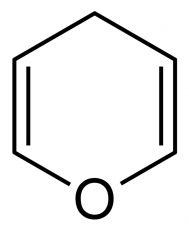
What is the common name for this ether? |
Pyran |
|
|
What are the 3 methods for naming oxiranes? |
1) Name the alkene it was derived from and add "oxide" eg. cyclohexane oxide
2) Treat the oxygen as a substituent named "epoxy". Use two numbers to specify its position
3) The oxirane ring is the parent. Oxygen is always #1, the carbons are 2 and 3 based upon alphabetical order of the substituents attached to them |
|
|
What are the 3 ways an ether can be synthesized? |
1) Fischer Esterification 2) Williamson Ether Synthesis 3) Alkoxymercuration-demercuration |
|
|
What two functional groups form an ester in a Fischer Esterification?
What is requires to catalyze the reaction?
How does this happen? |
Carboxylic acid and alcohol
Sulfuric acid is the catalyst
The H of the alcohol and the OH of the carboxylic acid come together in a dehydration synthesis |
|
|
How does a Williamson Ether Synthesis occur? What type of reaction is this?
What will happen if the carbon is not the correct degree? |
- An alkoxide ion attacks the primary carbon of an alkyl halide or tosylate - This is an Sn2 rxn and so the LG gets expelled in the same step
LG MUST be on a primary carbon. If the carbon is secondary or tertiary, the alkoxide will act as a base and an elimination will occur |
|
|
What are the reagents of an alkoxymercuration-demercuration? |
1st step: Hg(OAc)2 -------------- ROH
2nd step: NaBH4 |
|
|
What two reagents can be used to cleave an ether? Which is more effective?
What is the mechanism?
What are the products?
What happens if there is a phenol? |
HI > HBr
- Oxygen is protonated by the acid - the halogen (I or Br) Sn2 attacks the less substituted carbon and kicks out the alcohol and other side of what was the ether - The alcohol gets protonated and a substitution rxn with the halide occurs via either Sn1 or Sn2 depending on degree
Products are two alkyl halides
If there is a phenol there will not be a second substitution and there will be only one alkyl halide |
|
|
What are the two ways to synthesize an epoxide? |
1) MBPCA
2) |
|
|
If there are two double bonds in a molecule, which will be converted into the epoxide? |
The most electron rich double bond reacts faster AKA The more substituted bond |
|
|
What is base promoted halohydrin cyclization?
What is the mechanism?
What kind of base must be used |
When a hydroxy and halogen are located on the same molecule, and the alkoxide (hydroxy) displaces the halide ion to form a ring
1) A bulky base takes the H of the OH 2) The alkoxide ion Sn2 attacks the halogen's carbon, ejecting the halogen and forming a ring
A bulky base must be used to avoid a side rxn of a halide substitution to produce a diol |
|
|
What is the mechanism for acid-catalyzed opening of epoxides?
With what other reagents can this mechanism occur, forming what other products? |
1) The oxygen is protonated by the acid, leaving upon it a positive charge 2) Oxygen will pull electrons away from the more substituted carbon, giving the C a positive region 3) Water attacks this carbon and attaches with a positive charge 4) The water is deprotonated by more water, resulting in a trans diol
Can occur in alcohol solution to give an ether Can occur with hydrophilic acids to give a dialed (OH is displaced) |
|
|
What is different in the base-catalyzed opening of epoxides? |
The lower degree carbon is attacked instead of the higher one --> Sn2-like |
|
|
What are the 3 possible sets of reagents for this attack? |
1) NaOR / LiOR / KOR 2) Nitrogen containing 3) Organometallics |

