![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
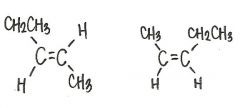
Are the two compounds shown below best describe as cis-trans isomers, constitutional isomers, or not isometric?
|
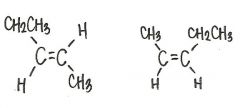
cis-trans isomers
|
|
|
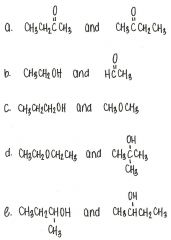
Which of the molecules below can hydrogen bond to another of the same compound?
a. (CH₃CH₂)2CHOH b. CH₃CH₂OCH₂CH₃ c. CH₃CH₂COCH₂CH₃ d. CH₃CH₂COOCH₃ e. all of the above |
a. (CH₃CH₂)2CHOH
|
|
|
What name is given to a hydrocarbon that contains a carbon-carbon triple bond?
|
a. alkyne
|
|
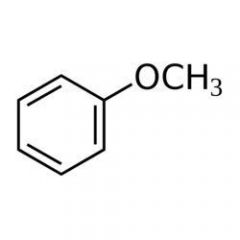
Anisole, the compound below , is an example of _____.
|
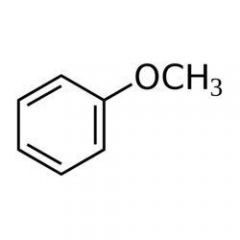
an ether
|
|
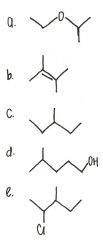
Which of the following has the highest boiling point?
add picture |
alcohols a higher boiling point
|
|
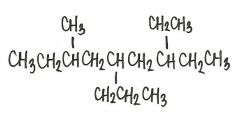
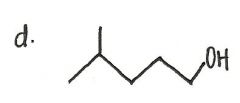
Provide an acceptable name for the alkane shown below.
|
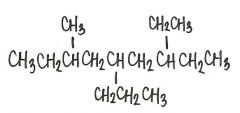
7-ethyl-3-methyl-5-propyl-nonane
|
|
|
By using the appropriate molecular formulas, write a balanced equation which describes the complete combustion of nonane.
|
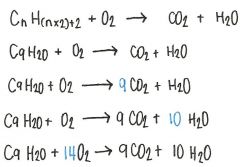
|
|
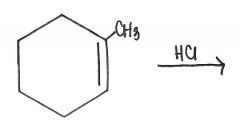
Draw the major organic product generated in the reaction below.
|
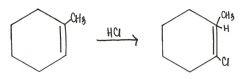
|
|
|
Oxidation of a 2° alcohol with chromic acid results in the production of _____.
|
a ketone
|
|
|
Oxidation of a 3° alcohol with chromic acid results in the production of _____.
a. an ester b. an aldehyde c. a ketone d. an ether e. none of the above |
e. none of the above
|
|
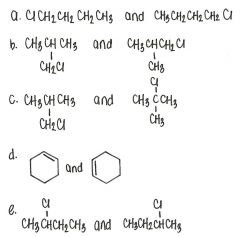
Which of the following pairs of compounds are structural isomers?
|
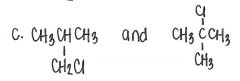
|
|
|
How many isomers are there for C₂H₄Cl₂?
|
two
|
|
|
Alkenes and alkyne are called unsaturated compounds because
|
they have fewer hydrocarbon atoms attached to the carbon chain than alkanes.
|
|
|
How many isomers are there for dibromobenzene?
|
two
|
|
|
Which of the following compounds is an alkyne?
a. CH₃CH₂C≣CH b. H₂C=CHCH=CH₂ c. 2-pentene d. C₃H₆ e. CH₃CH₂CH₃ |
a. CH₃CH₂C≣CH
|
|
|
When naming an alkene, the parent chain is the longest carbons chain ...
|
that contains both atoms of the double bond
|
|
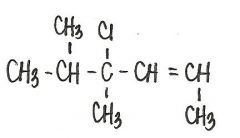
What is the IUPAC name for the following compound?
|
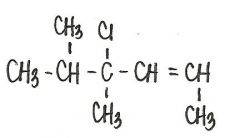
4-chloro-4,5-dimethyl-2-hexene
|
|
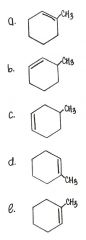
Which of the following is the correct structural formula for 3-methylcyclohexane?
|
|
|
|
Some alkenes have cis-trans isomers because ...
|
the carbon atoms in the double bond cannot rotate
|
|
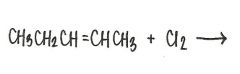
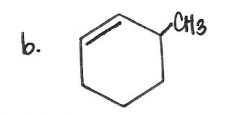
What is the product of this reaction?
|
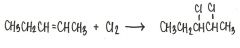
|
|
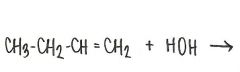
What is the major product of the reaction shown below?
|
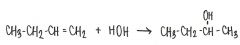
|
|
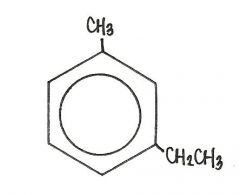
The name of the compound shown below is
|
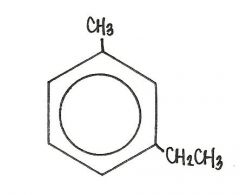
m-ethyltoluene
|
|
|
All of the carbon-carbon bonds in benzene are _____.
|
identical
|
|
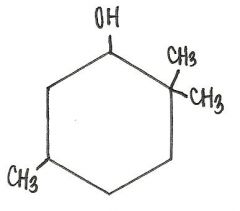
Which of the following is the IUPAC name for the compound below?
|
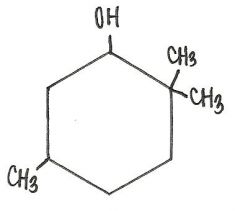
2,2,5-trimethylcyclohexanol
|
|
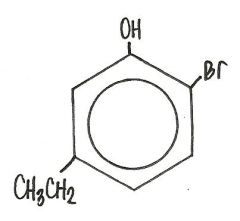
What is the name for this compound?
|
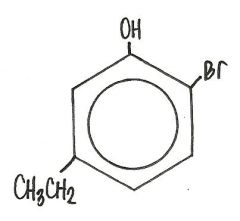
2-bromo-5-ethylphenol
|
|
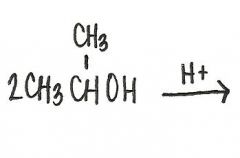
What is the structural formula of the ether formed in this reaction?
|
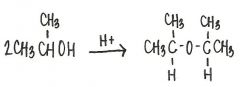
|
|
|
Tertiary alcohols cannot be oxidized because ...
|
there are no hydrogen atoms attached to the alcohol carbon
|
|
|
Thiols have structures similar to alcohols except that they contain
a. three alcohol groups b. lithium in place of oxygen in the functional group c. sulfur in place of oxygen in the functional group d. more than one carbon e. nitrogen in place of oxygen in the functional group |
c. sulfur in place of oxygen in the functional group
|
|
|
When a primary alcohol is completely oxidized, the product is a ________.
|
carboxylic acid
|
|
|
In the oxidation of an alcohol to a ketone, there is
a. a loss of carbon b. a loss of oxygen c. a loss of hydrogen d. a gain of hydrogen e. a gain of oxygen |
c. a loss of hydrogen
|
|
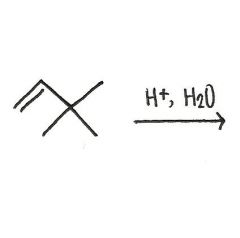
Draw the major organic product generated in the reaction below.
|
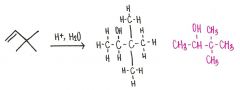
|
|
|
Compounds that have the same molecular formula but different arrangements of atoms are called _____.
|
isomers
|
|
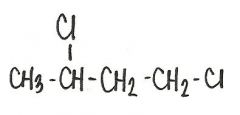
What is the name of this compound?
|
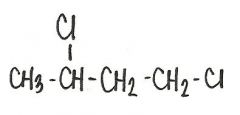
1,3-dicholorobutane
|
|
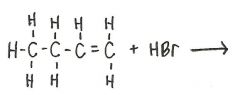
What is the product of the following reaction?
|
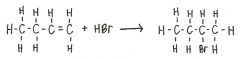
|
|
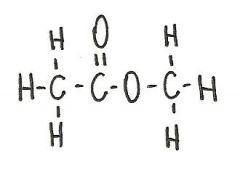
The compound below is a(n)
|
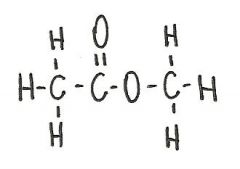
ester
|
|
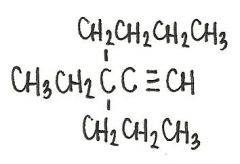
Name the following compound
|
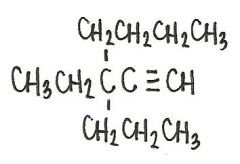
3-ethel-3-propyl-heptyne
|
|
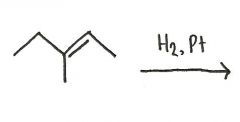
Draw the major organic product generated in the reaction below.
|
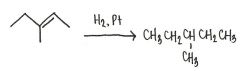
|
|
|
Benedict's test requires an aldehyde and an adjacent ______.
|
alcohol
|
|
Which of the following pairs of compounds are isomers?
|
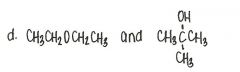
ester
|
|
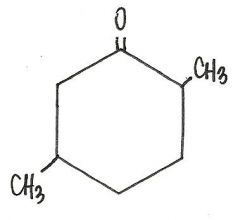
What is the IUPAC name for this compound?
|
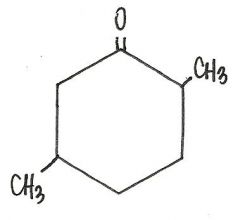
2,5-dimethylcyclohexanone
|
|
|
A formula that shows the arrangement of all bonds in a molecule is called a(n) ______.
|
complete structural formula
|
|
|
The functional group contained in the compound CH₃CH₂OH is a(n) ______.
|
alcohol
|
|
|
The functional group contained in the compound CH₃CH₂COOCH₃ is a(n) _____.
|
ester
|
|
|
The functional group contained in the compound CH₃CH₂SH is a(n) _____.
|
thiol
|
|
|
Identify the functional group in the compound CH₃CH₂N(CH₃)₂.
|
amine
|
|
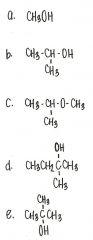
Which of the following compounds is a secondary alcohol?
|
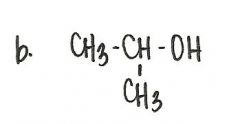
|
|
|
In a tertiary alcohol, how many alkyl groups are attached to the carbon atom bonded to the -OH group?
|
two
|
|
|
What is the IUPAC name for CH₃-CH₂-CH₂-SH?
|
1-propanethiol
|
|
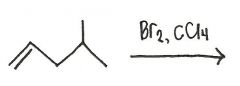
Draw the major organic product generated in the reaction below.
|
...
|
|
|
What is the acetal formed when propanone reacts with two molecules of methanol?
|
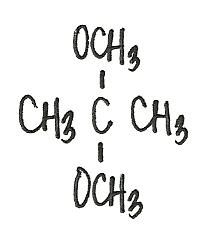
|

