![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Phenols
What are phenols? |
Organic compounds where an -OH group is directly attached to a benzene ring |
|
|
Phenols
At room temperature what state is phenol in? |
Solid state. |
|
|
Phenols
Describe the solubility of phenols |
Phenols are slightly soluble in water due to the presence of the OH group, which forms hydrogen bonds with H2O, but the benzene ring makes it less soluble. |
|
|
Phenols
What happens when phenols are dissolved in water? |
When dissolved in water, phenols form a weak acidic solution by losing H+ ions from the OH group. |
|
|
Phenols
What reactions of phenols should you know about? |
1) Sodium hydroxide
2) Sodium
3) Bromine |
|
|
Phenols
Write the word equation for the reaction between phenols and Sodium Hydroxide. |
Phenol + NaOH (aq) -> Salt + H2O
Phenols are neutralised by sodium hyrdroxide to form sodium phenoxide. |
|
|
Phenols
Draw the reaction between Sodium Hyrdroxide and Phenol. |
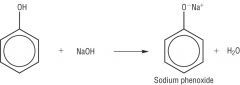
|
|
|
Phenols
Write the word equation for the reaction between phenols and Sodium |
Reactive metal (sodium) + Phenol -> Salt + H2 Gas |
|
|
Phenols
Draw the reaction between Phenol and Sodium |
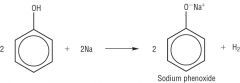
|
|
|
Phenols
Draw the reaction between a halogen and phenol. |
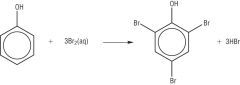
|
|
|
Phenols
Why do phenols have increased reactivity? |
Phenols have an increased reactivity because of the OH group. The p orbital lone electron pair on the oxygen atom is drawn into the ring which creates a high electron density. The ring isactivated.
This increased electron density is able topolarise the Bromine molecule which is then attracted more strongly to the ring than in benzene. |
|
|
Phenols
What are the uses of Alkyl Phenols? |
Surfactants and detergents |
|
|
Phenols
What are the uses of Chlorophenols? |
Antiseptics and disenfectants |
|
|
Phenols
What are the uses of Salycylic Acid? |
Used in the preperation of pharmaceuticals (aspirin) |
|
|
Phenols
What are the uses of Bisphenol? |
Used in the production of epoxy resins for paints. |

