![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Chromatography
Describe the process of chromatography |
1) Place the chromatography plate in the tank with a sample line at the bottom 2) Ensure the solvent level is below the sample line 3) Cover the tank and leave until the solvent front has reached the top of the plate 4) Remove the plate and mark the position of the solvent front |
|
|
Chromatography
Name some uses of chromatography |
Analysis of drugs, plastics, flavourings, foods, forensics, air samples, fuels etc |
|
|
Chromatography
What are some benefits of chromatography? |
Only small samples are needed After separation, the pure components can be analysed precisely using other analytical methods. |
|
|
Chromatography
What are the two types of chromatography you need to know? |
Thin-layer Gas |
|
|
Chromatography
Generally describe the 'science' behind chromatography |
A mobile phase sweeps a mixture over a stationary phase. It works on the basis that different components have different AFFINITIES for stationary phases and mobile phases: the stationary phase interacts with the components in the mixture, slowing them down. More interaction = more slowed down. This allows different components to flow over the stationary phase at different speeds, separating them. |
|
|
Chromatography
What is the state of the stationary and mobile phase in TLC? |
Stationary - Solid Mobile - Liquid |
|
|
Chromatography
What is the state of the stationary and mobile phase in GC? |
Stationary - Liquid/solid on solid support Mobile - Gas |
|
|
Chromatography
What does a solid stationary phase separate components by? Define. |
ADSORPTION - the process by which a solid holds molecules of a gas/liquid or solute as a thin film on the surface of a solid |
|
|
Chromatography
Draw diagrams to represent separation by adsorption and by relative solubility |
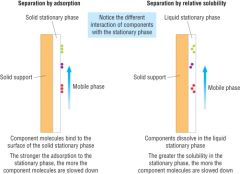
|
|
|
Chromatography
What is the stationary phase in TLC?
|
A thin layer of an adsorbant such as silica gel (SiO2) or Alumina (Al2O3) coated on a flat, inert support - a TLC plate, often a sheet of glass/plastic. |
|
|
Chromatography
What is the mobile phase in TLC? |
A liquid solvent. |
|
|
Chromatography
Rf = ? |
Rf = Distance moved by solvent / Distance moved by solvent front |
|
|
Chromatography
What can you use Rf values for? |
To identify an unknown compound by comparing known Rf values. |
|
|
Chromatography
What are the limitations of TLC? |
- Similar compounds often have similar Rf values - Unknown compounds will have no reference Rf to compare to - It may be difficult to find a solvent that separates all the components in a mixture- too soluble in solvent = washed up with solvent front little solubility = components will hardly move.
|
|
|
Chromatography
What is GC useful for? |
To separate volatile compounds - useful for compounds with low boiling points and evaporate easily. |
|
|
Chromatography
Describe the stationary and mobile phase in GC. |
STATIONARY - Thin layer of liquid/solid coated inside capillary tubing which acts as an inert solid support. Tubing (the chromatography column) is coiled so it fits in a thermostatically controlled oven. The liquid stationary phase is often a long-chain alkane with a high boiling point. Solid stationary phases include silicone polymers. MOBILE PHASE - Carrier gas which moves through the column. An inert or inactive gas such as He or N. |
|
|
Chromatography
Describe the process of GC. |
1) Mixture is injected into the gas chromatogram, where it is vaporised. 2) Mobile carrier gas flushes the mixture through the column 3) As the mixture moves through the column, components slow down as the interact with the stationary phase coating the column. (liquid = dissolve, solid = adsorb) Greater solubility/adsorption = more slowed down 4) Components separate and each leaves the column at a different time and is detected as it leaves, a computer processes these results. |
|
|
Chromatography
What is the retention time? + function |
The time for a component to pass from the column inlet to the detector.
Can be used to identify an unknown substance if compared with known retention times
|
|
|
Chromatography
What are the limitations of GC? |
- Many chemicals may have similar retention times, peak shape and detector response so GC might not identify them - Not all substances will be separated and detected - a small amount of a substance can hide beneath another that has a higher conc and the same retention time - Unknown compounds have no retention times for comparison |
|
|
Chromatography
Describe GCMS. |
GC - separates components, but can't identify them conclusively MS - provides detailed structural info on most compounds but cannot separate them
1)Components in a mixture are first separated by GC. Retention time provides an initial identification 2) Separated components are put in a MS where they are detected 3) MS is analysed or compared with a spectral database |
|
|
Chromatography
What is GCMS used for? |
Forensic and drug analysis, environmental analysis, airport security and space probes.
Forensics = analysis minute particles found at a crime scene. Environmental = Pollutants, quality of water, pesticides in food. Airports = Explosives Space = analyse atmosphere, material |
|
|
NMR
What is the purpose of NMR? |
Examining molecular structure. |
|
|
NMR
What is chemical shift? |
The place in an NMR specturm at which a nucleus absorbs energy |
|
|
NMR
What is chemical shift measured relative to? |
A reference signal from Tetramethylsilane TMS (CH3)4Si Chemical shift = 0 |
|
|
NMR
What is the problem with using a solvent for NMR? |
Organic solvents contain C and H which produce a peak themselves. |
|
|
NMR
What is used as solvents? |
Deuterated solvents are used as they do not produce a signal. |
|
|
NMR - CARBON 13
In C13 NMR, what does chemical shift indicate?
|
The chemical environments of the carbon atoms present. The presence of an electronegative element causes significant change in chemical shift. |
|
|
NMR - CARBON 13
In C13 NMR: a) Peaks show..? b) Chemical shifts show..? c) Size of peaks show..? |
a) Number of different carbon environments b) Types of carbon environments c) nothing. |
|
|
NMR- PROTON
In proton NMR: a) Number of peaks show..? b) Chemical shifts show..? c) Size of peaks show..? d) Splitting shows..? |
a) Number of proton environments b) Type of proton environment c) Relative peak area = proportions of protons in each environment d) Information of adjacent protons
|
|
|
NMR- PROTON
What is the N+1 rule? |
For n protons on an adjacent carbon, the number of peaks on a splitting pattern is N+1. |
|
|
NMR- PROTON
In proton NMR what happens when a deuterated solvent is added? |
Any peaks for OH and NH disappears because it exchanges with the H present in OH or NH. |
|
|
NMR- MEDICINE
Describe MRI. |
Patient is placed in a large, cylindrical electromagnet and radio waves are then sent through the body, Protons align with or against the strong magnetic field and they resonate in response to pulses of radio-frequency radiation. |
|
|
NMR - MEDICINE
What are the advantages of MRI? |
- Harmless -Non-invasive
|
|
|
NMR - MEDICINE
What are the disadvantages of MRI? |
- Some patients with ferromagnetic metal implants of pacemakers should not be subjected to MRI. |
|
|
NMR - MEDICINE
What are the uses of MRI in sport? |
Used to identify injuries. |
|
|
Combined techniques
What is the function of mass spec? |
determine molecular mass of a compound. |
|
|
Combined techniques
What is the function of IR? |
Gives information about the bonds present in a molecule and the likely functional groups present. |

