![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
Carboxylic Acids
What is the functional group of a carboxylic acid? |
COOH |
|
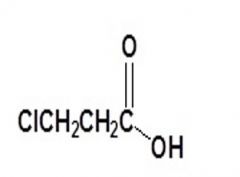
Carboxylic Acids
Name this compound: |
3-chloropropanoic acid |
|
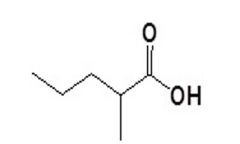
Carboxylic Acids
Name this compound: |
2-methylpentanoic acid |
|
|
Carboxylic Acids
Explain the solubility of Carboxylic Acids |
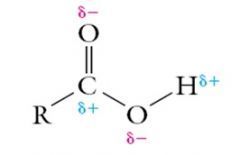
Carboxylic acids are very soluble in water due to the difference in electronegativities of Carbon and Oxygen. Hydrogen bonds form between water and the polar groups on a carboxylic acid.
|
|
|
Carboxylic Acids
What reactions of Carboxylic acids should you know? |
1) Reaction with metals
2) Reaction with metal carbonates
3) Reaction with bases |
|
|
Carboxylic Acids
Write the word equation for the reaction between a carboxylic acid and a metal. Include an example in your answer. |
Acid + Metal -> Salt + H2
CH3COOH + Na -> Ch3COO-Na+ + 1/2 H2 |
|
|
Carboxylic Acids
Write the word equation for the reaction between a carboxylic acid and a metal carbonate. Include an example in your answer. |
Acid + Metal Carbonate -> Salt + CO2 + H2O
2 CH3COOH + MgCO3 -> (CH3COO-)2Mg2+ + CO2 + H2O |
|
|
Carboxylic Acids
Write the word equation for the reaction between a carboxylic acid and a base. Include an example in your answer. |
Acid + Base -> Salt + Water
CH3COOH + NaOH -> CH3COO-Na+ + H2O |

