![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
105 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is a drug?
|
A chemical or biological agent that affects living processes.
|
|
|
|
What is clinical pharmacology? Toxicology?
|
It is the study of drug administration to and action on patients. Toxicology is the study of adverse effects of drugs.
|
|
|
|
Define pharmacodynamics (PD).
|
The study of what the drug does to the body. Includes study of drug actions, physiochemical actions, receptor interactions, pharmacological effects/side effects and therapeutic window/range. Key focus is on dose-response relationships and drug-receptor interactions.
|
|
|
|
Define pharmacokinetics (PK).
|
The study of what the body does to the drug. Includes study of drug absorption, distribution, metabolism and excretion/elimination (ADME). Key focus is plasma drug concentration as a function of time.
|
|
|
|
Define the term "receptor"
|
A receptor is the component of a cell or organism that interacts with drug and initiates the chain of events leading the drug's effects.
|
|
|
|
Describe the key tenets of Receptor Theory.
|
*Most receptors are proteins (i.e. regulatory proteins, enzymes, transport proteins, structural proteins, intracellular proteins)
*Receptors are part of the normal cell physiologic mechanisms and they interact with endogenous compounds (natural ligand) *Receptors are specific (re: ligand) *Receptors act as both ligand binder AND effector *Receptors may be stimulated (agonist) or inhibited (antagonist) *Drug-receptor interactions make small, accumulated changes in the cell that lead to changes in cell function. Tissues function is altered by the accumulated changes in cells of that tissue. *Maximal drug response (Emax) is related to the # drug-receptor interactions and the physiologic capacity of the tissue. *Structure of the drug determines its fit into the receptor; better fit means better receptor stimulation leading to pharmacological effect *Major theory assumption: each cell in a tissue contains a large population of receptors that are accessible to drugs. |
|
|
|
True or false: the cell membrane itself can function as a receptor?
|
True. Changes in electrical potential, membrane fluidity can act as receptor signals.
|
|
|
|
Describe the 6 major types of receptors
|
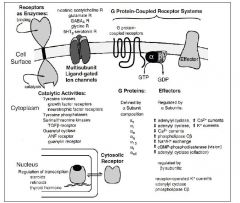
Membrane bound receptors (i.e. ion channels)
Enzymes--intracellular or extracellular Structural macromolecules (i.e. microtubules) Intracellular receptors (i.e. steroid receptors) Cell membrane |
|
|
|
Discuss the significance of drug-receptor bonds during their binding interactions.
|
Drug-receptor (DR) interactions are generally brief (fractions of seconds) so the bonds between them are usually reversible (including ionic, van der waals, hydrogen). This facilitates the activation of receptor/effect but also allows for termination of pharmacological effect. Conversely, irreversible or covalent binding is not common (unless used for antagonistic purposes).
|
|
|
|
Describe the mechanism of G protein coupled receptors in transducing and amplifying the drug receptor interaction.
|
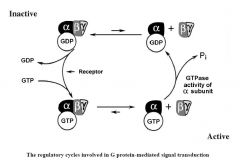
|
|
|
|
Define selectivity (of a drug).
|
The property of a drug to cause a specific effect. The primary effect is desired whereas the side effects may be undesired. Note: few drugs produce a single effect.
|
|
|
|
Define the Kd (dissociation constant).
|
A measure of the drug's affinity for a given receptor. The concentration of a drug required in solution to achieve 50% occupancy of its receptors.
The dissociation constant of a drug is equivalent to the EC50 and the 50%Emax. |
|
|
|
Define the EC50.
|
The concentration of a drug that induces the specified clinical effect in 50% of subjects.
|
|
|
|
Define intrinsic activity.
|
The intrinsic activity is the ability of the drug to stimulate the receptor once it is bound.
|
|
|
|
Define affinity (of drug).
|
Affinity of a drug refers to the strength of binding between a drug and its receptor.
|
|
|
|
Define efficacy (of drug).
|
Efficacy is the ability of the drug to activate the effector portion of the receptor once it is bound. Depends on the structure of a drug (re: intrinsic activity).
|
|
|
|
Define potency (of drug).
|
Potency of a drug relates to the amount of drug needed for effect; The amount of drug depends on the biologic system including receptor density, health of tissue, NRG status and stimulus response system.
|
|
|
|
Define antagonist.
|
Antagonist is a drug that can bind to the receptor but not produce a pharmacological response. In other words, it doesn't stimulate the receptor because it doesn't have the proper structure.
|
|
|
|
Define agonist.
|
A drug that binds to the receptor and produces a pharmacological response.
|
|
|
|
Define Emax
|
The maximal drug effect.
|
|
|
|
Alkylating Agents
|
---
|
Produce strong electrophiles through carbonium or ethyleneimonium ion intermediates, which covalently bond via alkylation of nucleophilic moieties in DNA (mostly N7 position of guanine);Cell Cycle Non-Specific (CCNS)
|
|
|
Ifosfamide (Ifex)
|
Nitrogen mustard
|
Alkylating agent;Prodrug; Conversion by hepatic cytochrome P450 to active metabolite phosphoramide mustard
|
|
|
Lomustine (Ceenu)
|
Nitrosoureas
|
Alkylating agent
|
|
|
Dacarbazine (DTIC)
|
Triazenes
|
Alkylating agent;<br />Prodrug; activated by liver cytochromes
|
|
|
Temozolomide (Temodar)
|
Triazenes
|
Alkylating agent;<br />Nonenzymatic conversion to methylhydrazine at physiologic pH
|
|
|
Cisplatin (Platinol)
|
Platinum analogs
|
Alkylating agents that do not form carbonium ion intermediates or formally alkylate DNA;Covalently bind nucleophilic sites on DNA (e.g., guanine N7);Converted to active cytotoxic forms by reacting with water to form (+)charged, hydrated intermediates that react with DNA guanine, forming inter- and intrastand cross-links
|
|
|
Carboplatin (Paraplatin)
|
Platinum analogs
|
Alkylating agents that do not form carbonium ion intermediates or formally alkylate DNA;<br />Covalently bind nucleophilic sites on DNA (e.g., guanine N7);<br />Converted to active cytotoxic forms by reacting with water to form (+)charged, hydrated intermediates that react with DNA guanine, forming inter- and intrastand cross-links
|
|
|
Oxaliplatin (Eloxatin)
|
Platinum analogs
|
Alkylating agents that do not form carbonium ion intermediates or formally alkylate DNA; <br>Covalently bind nucleophilic sites on DNA (e.g., guanine N7);<br>Converted to active cytotoxic forms by reacting with water to form (+)charged, hydrated intermediates that react with DNA guanine, forming inter- and intrastand cross-links
|
|
|
Antimetabolites
|
---
|
Structural analogs of folic acid or of the purine/pyramidine bases found in DNA;<br>Act in S-phase (CCS)
|
|
|
Methotrexate (Trexall)
|
Antimetabolite; Folate analogs
|
Inhibits dihydrofolate reductase (DHFR), which converts dietary folate to tetrahydrofolate (THF) needed for thymidine and purine synthesis;Given orally or intrathecally
|
|
|
Pemetrexed (Alimta)
|
Antimetabolite; Folate analogs
|
Active metabolite are polyglutamate forms that inhibit THF-dependent enzymes (e.g., DHFR, Thymidylate synthase (TS));
|
|
|
5-Fluorouracil (5-FU, Carac)
|
Antimetabolite; Pyramidine analogs
|
Prodrug; Converted to active metabolites:; ; ;5-FdUMP inhibits TS;; ; ;5-FdUTP incorporates into RNA & interferes with RNA function;
|
|
|
Capecitabine (Xeloda)
|
Pyramidine analogs
|
Prodrug converted to 5'-dFdU
|
|
|
Cytarabine (AraC, Depocyt)
|
Pyramidine analogs
|
Ara-C converted by deoxycytidine kinase to Ara-CMP --> Ara-CTP;Terminates DNA synthesis as Ara-CTP
|
|
|
Gemcitabine (dFdC, Gemzar)
|
Pyramidine analogs
|
Converted to active metabolites:; ; ;dFdCDP inhibits ribonucleotide reductase (lowers deoxyribonucleotide);; ; ;dFdCTP incorporates into DNA, terminating DNA synthesis
|
|
|
6-Mercaptopurine (Purinethol)
|
Purine analogs (antimetabolites)
|
Prodrug metabolized by hypoxanthine-guanine phosphoribosyl transferase (HGPRT) to 6-thioinosinic acid (TIMP);TIMP inhibits first step of de novo purine base synthesis and the formation of AMP and xanthinylic acid from inosinic acid, reducing purine levels;As well, TIMP is converted to thio-guanine ribonucleotides, inhibiting DNA and RNA synthesis
|
|
|
DNA Intercalating Agents
|
---
|
Bind DNA through intercalation between specific bases, blocking DNA, RNA or both synthesis;Cause DNA strands to break and interfere with cell replication;CCNS
|
|
|
Dactinomycin (Actinomycin D, Cosmegen)
|
Antitumor antibiotic; DNA Intercalating agent
|
Intercalates G-C base pairs of DNA; interfering with DNA-dependant RNA polymerase (inhibits DNA transcription);;Also causes ssDNA breaks
|
|
|
Daunorubicin (Cerubidine)
|
Anthracyclines
|
Intercalate between DNA base pairs and donate electrons to O2 to form superoxide;Superoxide reacts with itself to form H2O2 --> cleaved in the presence of Fe to form OH radical, which cleaves DNA
|
|
|
Idarubicin (Idamycin)
|
(antitumor antibiotic); Anthracyclines; DNA Intercalating agent
|
Intercalate between DNA base pairs and donate electrons to O2 to form superoxide;<br>Superoxide reacts with itself to form H2O2 --> cleaved in the presence of Fe to form OH radical, which cleaves DNA
|
|
|
Doxorubicin (Doxil)
|
(antitumor antibiotic); Anthracyclines; DNA Intercalating agent
|
Intercalate between DNA base pairs and donate electrons to O2 to form superoxide (ROS);<br />Superoxide reacts with itself to form H2O2 --> cleaved in the presence of Fe to form OH radical, which cleaves DNA
|
|
|
Epirubicin (Ellence)
|
Anthracyclines
|
Intercalate between DNA base pairs and donate electrons to O2 to form superoxide;<br>Superoxide reacts with itself to form H2O2 --> cleaved in the presence of Fe to form OH radical, which cleaves DNA
|
|
|
Mitoxantrone (Novantrone)
|
(antitumor antibiotic); Anthracyclines; DNA Intercalating agent
|
Intercalate between DNA base pairs and donate electrons to O2 to form superoxide;<br />Superoxide reacts with itself to form H2O2 --> cleaved in the presence of Fe to form OH radical, which cleaves DNA
|
|
|
Bleomycin (Blenoxane)
|
antitumor antibiotic; DNA Intercalating agent
|
Binds to DNA, contributes to free radical formation producing ss- and dsDNA breaks
|
|
|
Microtubule Inhibitors
|
---
|
Inhibit mitosis and cause metaphase arrest by interfering with microtubule function (tubulin (de)polymerization);<br>CCS
|
|
|
Vinblastine
|
Vinca alkaloids
|
Block tubulin polymerization into microtubules
|
|
|
Vincristine (Oncovin)
|
Vinca alkaloids; Microtubule inhibitor (MTI)
|
Block tubulin polymerization into microtubules; CCS: M-phase (mitotic arrest)
|
|
|
Paclitaxel (Abraxane);
|
Taxanes; Microtubule Inhibitor (MTI)
|
Block microtubule depolymerization into tubulin; CCS: M-phase
|
|
|
Docetaxel (Taxotere)
|
Taxanes; Microtubule Inhibitor
|
Block microtubule depolymerization into tubulin
|
|
|
Topoisomerase Inhibitors
|
---
|
Prevent the resealing of topo I (ssDNA) and topo II (dsDNA);CCS
|
|
|
Etoposide (Etopophos)
|
Epipodophyllotoxins
|
Inhibits topoisomerase II
|
|
|
Teniposide (Vumon);
|
Epipodophyllotoxins; Topoisomerase Inhibitor(TI)
|
Inhibits topoisomerase II; results in DNA damage
|
|
|
Irinotecan (Camptosar)
|
Camptothecin analogs
|
Inhibits topoisomerase I
|
|
|
Topotecan (Hycamtin)
|
Camptothecin analogs; Topoisomerase Inhibitor
|
Inhibits topoisomerase I; results in DNA damage
|
|
|
Hormone Therapy
|
---
|
Treatment of hormone-dependent neoplasms
|
|
|
Prednisone (Meticorten)
|
Glucocorticoids
|
Inhibit mitosis in lymphocytes
|
|
|
Dexamethasone (Decadron)
|
Glucocorticoids
|
Inhibit mitosis in lymphocytes
|
|
|
Tamoxifen (Soltamox)
|
Selective estrogen-receptor modulators (SERMs)
|
Competes with estradiol for binding to estradiol receptor
|
|
|
Fulvestrant (Faslodex)
|
Selective estrogen-receptor downregulators (SERDs)
|
Binds with much higher affinity (>100-fold) to estrogen receptor than tamoxifen, inhibiting dimerization, increasing degradation, and reducing overall ER levels
|
|
|
Aminoglutethamide (Cytadren)
|
Aromatase inhibitors
|
Inhibit function of aromatase (converts androstenedione and testosterone to estrone and estradiol)
|
|
|
Anastrozole (Arimidex)
|
Aromatase inhibitors
|
Inhibit function of aromatase (converts androstenedione and testosterone to estrone and estradiol)
|
|
|
Letrozole (Femara)
|
Aromatase inhibitors
|
Inhibit function of aromatase (converts androstenedione and testosterone to estrone and estradiol)
|
|
|
Exemestane (Aromasin)
|
Aromatase inhibitors
|
Steroidal inhibitor of aromatase
|
|
|
Leuprolide (Lupron)
|
GnRH analogs
|
Binds GnRH receptor; inhibits release of FSH & LH
|
|
|
Goserelin (Zoladex)
|
GnRH analogs
|
Binds GnRH receptor; inhibits release of FSH & LH
|
|
|
Flutamide (Eulexin)
|
Nonsteroidal androgen-receptor blockers
|
Competes with androgen for AR binding
|
|
|
Bicalutamide (Casodex)
|
Nonsteroidal androgen-receptor blockers
|
Complete with receptor for hormone
|
|
|
Imatinib (Gleevac)
|
Tyrosine Kinase inhibitor
|
Inhibits Abl kinase by binding where ATP should go;Also inhibits PDGFR and c-kit;Metabolized by cytochrome P450
|
|
|
Gefitinib (Iressa)
|
Tyrosine Kinase inhibitor
|
Inhibit epidermal growth factor receptor (EGFR) tyrosine kinase
|
|
|
Erlotinib (Tarceva)
|
Tyrosine Kinase inhibitor
|
Inhibit epidermal growth factor receptor (EGFR) tyrosine kinase
|
|
|
Nilotinib (Tasigna)
|
Tyrosine Kinase inhibitor
|
Inhibits Abl kinase;
|
|
|
Dasatinib (Sprycel)
|
Tyrosine Kinase inhibitor
|
Inhibits Abl & Src kinases
|
|
|
Rituximab (Rituxan)
|
Monoclonal antibody
|
CD20 B-cell antibody that can directly activate apoptosis, activate complement, or activate cell-mediated cytotoxicity (e.g., T cells, NK cells)
|
|
|
Trastuzumab (Herceptin)
|
Monoclonal antibody
|
Unknown HER2/neu (ErbB2) receptor antibody mechanism (enhanced receptor endocytosis or blocking homo- or heterodimerization)
|
|
|
Cetuximab (Erbitux)
|
Monoclonal antibody
|
EGFR1 (ErbB1)
|
|
|
Hydroxyurea (Hydrea)
|
---
|
Inhibits ribonucleoside diphosphate reductase
|
|
|
Retinoids
|
---
|
ATRA induces terminal differentiation in malignant immature promyelocytes, which subsequently apoptose
|
|
|
Arsenic Trioxide (Trisenox)
|
---
|
---
|
|
|
Thalidomide (Thalomid)
|
---
|
---
|
|
|
Interferons
|
---
|
---
|
|
|
Asparaginase (Elspar)
|
---
|
Deaminates asparagine --> inhibition of protein synthesis
|
|
|
ABVD
|
Combination therapy
|
Doxorubicin (adriamycin), bleomycin, vinblastine, dacarbazine
|
|
|
CHOP
|
Combination therapy
|
Cyclophosphamide, hydroxydoxorubicin, vincristine (oncovine), prednisone
|
|
|
MOPP
|
Combination therapy
|
Mechlorethamine, vincristine (oncovine), procarbazine, prednisone
|
|
|
CMF
|
Combination therapy
|
Cyclophosphamide, methotrexate, 5-fluorouracil
|
|
|
FEC
|
Combination therapy
|
5-fluorouracil, epirubicin, cyclophosphamide
|
|
|
Ipilimumab (Yervoy)
|
Human monoclonal antibody
|
Cytotoxic T-Lymphocyte Antigen 4 inhibitor; stimulates immune system
|
|
|
Vemurafenib (Zelboraf)
|
Serine/threonine kinase inhibitor
|
Inhibits oncogenic BRAF kinase
|
|
|
Dabrafenib (Tafinlar)
|
Serine/threonine kinase inhibitor
|
Inhibits oncogenic BRAF kinase
|
|
|
Trametinib (Mekinist)
|
---
|
Inhibits MEK
|
|
|
List the 6 mechanisms of acquired drug resistance.
|
1. Decreased cellular uptake2. Abnormal transport of the drug (i.e. efflux by P-glycoprotein)3. Increased cellular activation (binding/metabolism)4. Altered target protein5. Reduced affinity for the drug6. Enhanced repair of DNA damage
|
|
|
|
List the 7 most common side effects of chemotherapy.
|
1. Neutropenia2. Thrombocytopenia3. Anemia4. Nausea & Vomiting5. Stomatitis6. Alopecia (hair loss)7. Secondary leukemia (due to long term CTX tx)
|
|
|
|
List the 4 subclasses of alkylating agents.
|
1. Nitrogen mustards2. Nitrosoureas3. Triazenes4. Platinum analogs
|
|
|
|
List the 3 Nitrogen mustards
|
1.Mechlorethamine2. Cyclophosphamide3. Ifosfamide
|
Alkylating
|
|
|
List the 2 Nitrosoureas drugs
|
1. Carmustine2. Lomustine
|
Alkylating
|
|
|
List the 2 Triazenes
|
1.Dacarbazine2. Temozolomide
|
Alkylating
|
|
|
List the 3 Platinum analogs
|
1. Cisplatin2. Carboplatin3. Oxaliplatin
|
Alkylating
|
|
|
For;Mechlorethamine (Mustargen):;1. Pharmacologic class/subclass
|
Alkylating agent; Nitrogen mustard
|
|
|
|
For Mechlorethamine (Mustargen):-Indicate its MOA
|
--
|
Spontaneously converted to active metabolites in body fluids or enzymatically converted to metabolites in liver.;
|
|
|
Cyclophosphamide (Cytoxan)--Indicate pharmacological class/subclass
|
Alkylating agent; Nitrogen mustard
|
|
|
|
Cyclophosphamide (Cytoxan)--Indicate MOA
|
Prodrug; Converted to active metabolite phosphoramide mustard by hepatic cytochrome P450 enzymes. Alkylates DNA;
|
|
|
|
Ifosfamide (Ifex):--Indicate pharmacological class/subclass
|
Alkylating agent; Nitrogen mustard
|
|
|
|
Ifosfamide (Ifex):--Indicate MOA
|
Prodrug; MOA same as cyclophosphamide
|
|
|
|
Carmustine (Gliadel):--Indicate pharmacological class/subclass
|
Alkylating agent; Nitrosoureas
|
|
|
|
Carmustine (Gliadel):--Indicate MOA
|
Alkylating agent; highly lipid-soluble; can cross BBB
|
|

