![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
291 Cards in this Set
- Front
- Back
|
Define Pathology
|
Study of disease
Functional, structural, biochemical alterations in the body produce disease |
|
|
Define Epidemiology
|
WHO tends to be affected by a disease process
|
|
|
Define Homeostasis
|
Ability of body to maintain a condition
|
|
|
Define Allostasis
|
"Other", Differing, Varrying = maintain some degree of stability resulting from a change
|
|
|
Define Allostatic Overload
|
When continued stress is over-whelming and allostasis can't be upheld
|
|
|
T/F: Pathological specimens can tell us information about a period of time within an organism
|
FALSE. Pathological specimens represent a "snap shot" in time of body structure
|
|
|
What are the two main divisions of pathology?
|
Anatomic and Clinical
|
|
|
List some examples of Anatomic Pathology
|
Surgical Pathology
Cytology Autopsy/Forensic Pathology |
|
|
List some examples of Clinical Pathology (Laboratory Medicine)
|
Microbiology/Virology
Clinical Chemistry Blood Bank/Transfusion Medicine/Hematology HLA |
|
|
What is the central dogma of molecular biology?
|
DNA --> RNA --> Protein
|
|
|
Define what is meant by risks in the central dogma of health and disease
|
Risks = Stressors that challenge Homeostasis (for ex. when chemicals in smoke cause cancer, they become etiological)
|
|
|
When do we want to intervene with patients?
|
Before risks cause disease, because after that time we have to TREAT disease = we want to stay away from having to treat
|
|
|
What are the two big causes of disease and Differential Diagnoses?
|
Nature (Genetics) and Nurture (Environment)
|
|
|
What are the categories of disease?
|
VINDICATE
Vascular Infectious/Inflammatory Neoplastic (primary/secondary) Drugs Idiopathic/Latrogenic (=we don't know) Congenital/Developmental/ Inherited Autoimmune/Allergic/ Anatomic Trauma Endocrine/Metabolic/Environmental/ Occupational |
|
|
What types of cellular responses to stimuli are Pathologic? What types of cellular responses are physiologic?
|
Pathologic:
Injury Death (Apoptosis or Necrosis) Accumulations Physiologic: Adaptations |
|
|
Detail the events if a cell is exposed to a stressor for a prolonged period of time
|
Cell exposed to stressor
Cell Adapts to respond to stressor Cell structure and function are altered Cell Injury and ultimately cell death may occur |
|
|
What are the 4 major types of Adaptations cells undergo?
|
"HHAM"
Hyperplasia (Inc. Cell Number) Hypertrophy (Inc. Size of Cell...think of a "big" Trophy) Atrophy (Dec. Size of cell) Metaplasia (change from one mature cell type to another...think "meta"morphosis) |
|
|
What is the goal of cellular adaptation?
|
to adjust to new conditions/demands
Reflects dynamic ability of cells to alter their cell cycle activity |
|
|
Define Hyperplasia
|
Inc. Number of Cells
Requires DNA synthesis and cell division |
|
|
Why might Hyperplasia occur Physiologically (i.e. normally)?
|
Hormonal = If we need more hormones
Compensatory = we need to regain some functional capacity after losing some cells |
|
|
Why might Hyperplasia occur Pathologically?
|
Cells might proliferate for protection...
For Ex., Epithelial Hyperplasia might occur to thicken epidermis to protect us |
|
|
Give some examples of Physiological Hyperplasia and Pathological Hyperplasia
|
Physiological = partial liver removal = remaining liver undergoes hyperplasia growth
Endometrial thickness bc of estrogenic stimulation (proliferative phase) Pathologic = Endometrial Hyperplasia (i.e. Endometriosis!) Psoriasis or Lichen Simplex = epidermal hyperplasia |
|
|
Define Hypertrophy
|
Increase in the size of an organ because the cells increase in size (bc of increased protein synthesis)
|
|
|
T/F: Hypertrophy generally occurs in cells that would not typically divide
|
TRUE (muscle cells, cardiac cells)
|
|
|
What happens when the heart undergoes cardiac hypertrophy?
|
Cells in heart inc. in size = Left Ventricle Thickens = it can't hold as much blood = Decreased volume capacity of Left Ventricle = we'll need to inc. Heart Rate to compensate
|
|
|
Define Atrophy
|
A decrease in cell size because of a loss of cellular substance
Occurs because the cellular environment can't support the cell's current size, so it shrinks (This is what occurred in the movie "Honey, I Shrunk the Kids") Decreased cell size = decreased function |
|
|
What are the causes of atrophy?
|
"DDRIP"
Decreased workload (disuse) Denervation (loss of nerve supply) Reduced endocrine stimulation Ischemia Poor Nutrition |
|
|
Define Metaplasia
|
Change of cell from one mature type to another
For example: squamous epi --> Columnar epi |
|
|
What is thought to cause Metaplasia?
|
Reprogramming of gene expression of stem cells in tissue
Generally is protective to noxious stimuli (change from susceptible cell type to more resistant/protective cell type) |
|
|
T/F: Typically, Metaplasia is Irreversible
|
FALSE.
Metaplasia is typically REVERSIBLE |
|
|
Define Dysplasia
|
If injurious stimulus continues to affect cell, progressive changes may result in dysplasia = one step before cell becomes neoplastic and pre-cancerous
|
|
|
List the 7 causes of cell Injury
|
"PIINCH - G"
Physical Agents (mechanical agents like temp. change) Infectious Agents Immunologic Reactions Nutritional Imbalance Chemicals/Drugs Hypoxia (= diminished O2 delivery vs. Ischemia = Impaired blood flow) Genetic Mutations |
|
|
What are the underlying mechanisms of cell injury?
|
Ox. Phos. (dec. ATP)
Ca2+ Homeostasis ROS (Reactive Oxygen Species) Cell Membranes (inability to maintain membrane integrity) |
|
|
What are the consequences of Ox. Phos. Disruption?
|
For ex. Ox. Phos. disruption may occur because of ischemia.
dec. Ox. Phos. = Mito less effective = Dec. ATP = many, many cellular processes affected |
|
|
What are some of the pathological effects of ROS build up?
|
ROS react with:
1. FAs --> disruption of plasma membrane 2. Proteins --> Loss of activity, abnormal folding 3. DNA --> mutations, breaks |
|
|
List some of the Antioxidant mechanisms that contribute to removal of free radicals
|
1. SOD (O2--> H2O2)
2. Glutathione (OH--> H2O and O2) 3. Catalase (H2O2 --> H2O and O2) |
|
|
T/F: The severity and duration of causes of cell injury determine reversibility of cell damage
|
TRUE
|
|
|
T/F: most cells are already dead by the time we see the effects of the cell death
|
TRUE
|
|
|
T/F: Cell damage is reversible along a period of time as stressor continues
|
FALSE. Cell damage is reversible up to a specific point as stressor continues, but beyond that point cell damage is irreversible
|
|
|
What are some examples of reversible cell injury?
|
Cell swelling
Fatty change (accumulation of lipid vacuoles) |
|
|
Irreversible cell injury leads to what?
|
Cell Death
|
|
|
Whats the difference between necrosis and apoptosis?
|
Necrosis = ONLY PATHOLOGIC. membrane becomes leaky = leakage of cellular proteins and constituents = can further damage neighboring cells.
Apoptosis = CAN BE PHYSIOLOGIC = NORMAL. programmed cell death. Cellular fragmentation occurs |
|
|
How can Reperfusion injury cause cell death?
|
Generating ROS
Inflammatory Mechanisms |
|
|
How will DNA damage between apoptosis and necrosis look different on a DNA gel?
|
Apoptosis = cellular fragmentation = DNA in-tact, just in different size = gel will look like a ladder
Necrosis = DNA gel will show a continuous smear of DNA b/c DNA fragments are not distinct sizes. |
|
|
T/F: Nuclear chromatin condensation occurs in both apoptosis and necrosis
|
TRUE
|
|
|
T/F: Nuclei Shrink, fragment, and dissolve in apoptosis and necrosis
|
FALSE.
DNA shrink and fragment in both, but its only in necrosis that DNA dissolve |
|
|
T/F: Membrane integrity is upheld in apoptosis
|
TRUE
Membrane-bound cell fragments "bleb" off |
|
|
Which involves a larger area of tissue, apoptosis or necrosis?
|
necrosis
Apoptosis involves isolated cells or small groups of cells |
|
|
Which involves an inflammatory response, necrosis or apoptosis?
|
necrosis
Apoptosis does not involve an inflammatory response (makes sense, since it occurs naturally = programmed cell death) |
|
|
What are the 4 types of necrosis?
|
Coagulative
Caseous Liquefactive Fat Necrosis |
|
|
Define Coagulative necrosis
|
Denaturation of cytosolic proteins
Basic cell outline preserved = OVERALL ARCHITECTURE MAINTAINED Shrunken, increased eosinophilia (dec. pH) Pyknosis (nuclear shrinkage/darkening) Karyolysis (nuclear dissolution) Karyorrhexsis (fragmentation of nucleus) Nuclei dissapear within a few days |
|
|
Define Liquefactive Necrosis
|
Complete enzymatic digestion of dead cells
ex. = bacterial and fungal infections Pus. ew. Tissue Architecture LOST (no cell outlines when looking at in under microscope) |
|
|
Define Caseous Necrosis
|
Cheesy, white/yellow crumbly (friable) material produced
No Tissue Architecture Surrounded by "granulomatous" inflammation Seen in tuberculosis |
|
|
What types of necrosis preserve cell architecture? (Coagulative, Caseous, Liquefactive, Fat Necrosis?)
|
Coagulative
|
|
|
Define Fat Necrosis
|
Destruction of Adipocytes in fat tissue
Large scale destruction can lead to soponification = where soap is created = deposition of calcium salts in tissue (as fat cells die, they release Ca2+) |
|
|
Whats the difference between "dry" and "wet" Gangrenous Necrosis
|
Dry = Coagulative necrosis secondary to profound ischemia
Wet = Liquefactive necrosis from inflammatory necrosis superimposed on ischemic necrosis (secondary to bacterial infection at site of ischemic damage) |
|
|
List some Physiological examples of Apoptosis in the body
|
Involution of structures during development
Elimination of immune cells Involution following hormonal withdrawal (ex. = menstruation) Cytotoxic T cell mediated elimination of infected cells or neoplastic cells |
|
|
List some Pathological examples of Apoptosis in the body
|
"Insults" i.e. radiation, drugs, DNA damage
Viral Infections Autoimmunity Neoplastic cells |
|
|
Where do both the intrinsic and extrinsic stimulus pathways lead in apoptosis?
|
They lead to the common execution pathway, via Caspase Enzyme Cascade
|
|
|
What are the two ways Apoptosis can occur, and list some examples
|
Intrinsic = radiation, toxins, ROS free radicals, withdrawal of growth factors
Extrinsic = Death receptors: Fas (CD95), FasL (Fas Ligand CD95L) TNFR1 and TNF, Cytotoxic T-cell-mediated = Granzyme B Note: Pretty sure this slide is backwards in terms of intrisic and extrisic. |
|
|
What are some examples of Pro-Apoptotic Bcl Family mediators?
|
Pro-Apoptotic - Kill Cell
Bax (think of Katie Goodin's Dog, Bax, who killed rabbits for fun, bastard) Bak |
|
|
What are some examples of Anti-Apoptotic Bcl Family mediators?
|
Anti-Apoptotic = Cell Lives
bcl-2 bcl-x |
|
|
What does P53 do?
|
Its a tumor suppressor gene; part of the INTRINSIC Apoptosis pathway
Arrests cell in G1 to repair DNA If unsuccessful repair - apoptosis |
|
|
How do you alter the Bcl family of proteins to FAVOR Apoptosis?
|
Favor Apoptosis = Inc. Pro-Apoptotics =
Inc. Bax and Bcl Dec. bcl-2 and bcl-x |
|
|
How do all stimulus pathways get cells to die via apoptosis?
|
Caspases (enzyme cascade)
Disruption of the balance of pro-apoptosis and anti-apoptosis can lead to disease |
|
|
Too Little Apoptotic activity can lead to what?
|
Cancers
Autoimmunity |
|
|
Too Much Apoptotic activity can lead to what?
|
Neurodegenerative disorders (for ex. Parkinson's)
Ischemic Injury Death of virally infected cells |
|
|
T/F: Accumulations can be Intracellular or Extracellular
|
TRUE
|
|
|
What are accumulations a manifestation of?
|
metabolic derangement
|
|
|
What are some of the normal Accumulations in cells?
|
Fatty Acids (Abnormal = steatosis = fatty liver from alcohol toxicity)
Cholesterol (atherosclerosis, xanthomas) |
|
|
Is Steatosis (=fatty liver) reversible?
|
Yes
|
|
|
What are Xanthelasmas?
|
Lipid-laden macrophages
50% associated with hypercholesterolemia |
|
|
List some other accumulations
|
Proteins
Glycogen Pigments (Exogenous ex. = carbon dust, coal, smog) Endogenous ex. = Melanin, Lipofuscin (wear/tear pigment that does NOT stain with iron), Hemosiderin (Hemolytic break down product) |
|
|
What is Lipofuscin?
|
A "wear and tear" pigment that accumulates intracellular
Not Pathological |
|
|
What is Hemosiderin Pigment?
|
Iron-containing pigment that is a breakdown product of Hb
We can see it via microscope with iron stain |
|
|
What are some Pathological Calcifications?
|
Dystrophic Calcification
Metastatic Calcification |
|
|
Define Dystrophic Calcification
|
Ca2+ deposited abnormally in tissues (normal systemic Ca2+ levels)
Associated with dying or degenerating cells Ex. = calcific atherosclerosis and valvular disease (aortic stenosis bc of lots of cell death in that area = stimulus for dystrophic calcification), fat necrosis, certain neoplasms (Psammoma body) |
|
|
What are some causes of Metastatic Calcification?
|
Inc. PTH (for various reasons) = Hypercalcemia = inc. serum Ca2+
Bone destruction (bc of tumors, other reasons) = inc. Ca2+ Too Much Vitamin D |
|
|
T/F: Metastatic calcification may occur widely throughout the body
|
TRUE
Typically involves Gastric Mucosa, Kidneys, Lungs, Blood Vessels |
|
|
Another name for cellular aging is?
|
Cellular Senescence
|
|
|
Whats the concept of replicative senescence?
|
cells have a limited ability to replicate (telomeres etc.)
|
|
|
T/F: As cells age, there is declining function of the proteosome
|
TRUE
= cellular machinery that eliminates unwanted or abnormal proteins within the cell |
|
|
What are some Initiators of inflammation?
|
1. Infections
2. Trauma 3. Physical or Chemical Agents 4. Tissue Necrosis 5. Foreign Bodies 6. Hypersensitivity Immune Reactions |
|
|
What are the cardinal features of inflammation?
|
heat, redness, swelling, pain
|
|
|
What are the general responses to Inflammation?
|
Vascular
Activation of Chemical Mediators Migration and Activation of Cells (WBCs) Termination/Resolution |
|
|
How does acute inflammation differ from chronic inflammation?
|
Acute = Immediate, Rapid onset, short, sequential, innate immunity, neutrophils predominate
Chronic = Slower response/onset, cell mediated, lymphocytes/plasma cells/macrophages predominate, tissue destruction with granulation tissue and fibrosis |
|
|
What are the Components of Inflammation?
|
1. Vasculature (Fluid, Chemical, Cells)
2. Connective Tissue (Matrix, Cells) 3. Parenchyma = the cell type that performs the major function in that tissue |
|
|
When you alter the hemodynamics of a tissue, what is occurring?
|
Vasoconstriction and Vasodilation (redness/heat)
|
|
|
What is the net effect of vasodilation?
|
Increased Hydrostatic Pressure
Increased Interstitial Fluid (edema) |
|
|
Contraction of endothelial cells leads to:
A. Decreased vascular permeability B. Increased vascular permeability |
Contraction of endothelial cells --> Increased Vascular Permeability --> Increased Interstitial fluid and cells that exit vessels (extravasation)
|
|
|
Define Transudate
|
Fluid leaking out of vessels due to increased venous pressure
|
|
|
Define Exudate
|
Increased vascular permeability due to forces that push fluid across endothelial barrier. Will contain a high protein concentration relative to transudate
|
|
|
T/F: A Transudate condition means increased Hydrostatic Pressure and Decreased Colloid Osmotic Pressure
|
TRUE
Transudate = extravascular fluid with LOW protein content (FLUID escapes vessel) vs. Exudate = extravascular fluid with HIGH protein content (fluid AND protein escape vessel) |
|
|
How do hydrostatic pressure and colloid osmotic pressure change in an exudate condition?
|
Decreased
|
|
|
What two things occur during the Exudate process to allow inflammation to occur?
|
Vasodilation and Stasis
Increased Interendothelial Spaces (=allows the fluid and protein to leak out) |
|
|
T/F: The Transudate condition is synonymous with a non-inflammatory state
|
TRUE
|
|
|
Transudate and Exudate are associated with what vascular states?
|
Transudate = Non-inflammatory
Exudate = Inflammatory |
|
|
Which is associated with a higher protein content, and thus a higher specific gravity, transudate or exudate?
|
Exudate
|
|
|
Define Effusion
|
Excess fluid in body cavities
(ex. = peritoneal, pericardial, pleural) |
|
|
Define Serous
|
Yellow, Straw-like color, few cells
|
|
|
Define Serosanguinous
|
RBC's (red tinge)
|
|
|
Define Fibrinous
|
Large amounts of fibrin due to activation of coagulation cascade
|
|
|
Define Purulent
|
Large numbers of PMNs = white blood cells (neutrophils)
|
|
|
What are the origins of Endogenous Chemicals that activate and amplify the process of inflammation?
|
Plasma-derived
Cell-derived ECM (extracellular Matrix) |
|
|
Histamine, a principle chemical mediator of inflammation, is derived from what cells?
|
Mast cells, basophils, Platelets
|
|
|
Serotonin, a principle chemical mediator of inflammation, is derived from what cells?
|
Platelets
|
|
|
What are the newly synthesized mediators of inflammation in a cell undergoing inflammation?
|
Prostaglandins
Leukotrienes Platelet-activating factor ROS Nitric Oxide Cytokines Neuropeptides |
|
|
What products, newly synthesized during the inflammation process, are common targets for drugs?
|
Prostaglandins and Leukotrienes
|
|
|
Why does the body circulate inflammatory factors in their inactive form vs. their active form?
|
So we don't have inflammation all the time
|
|
|
What factors are activated from compliment activation that participate in the inflammatory process?
|
C3a, C5a, C3b, C5b-9 (membrane attack complex MAC)
|
|
|
What does Factor XII (12) activate?
|
Kinin System (Bradykinin)
Coagulation/ Fibrinolysis system (=breaks down fibirn clot) |
|
|
T/F: The Compliment System involves 20-30 dif. proteins and 2 or 3 pathways.
|
TRUE
Classical - Ab-Ag Complexes Alternative - Triggered by complex molecules = bacterial lipopolysacchardies (LPS) |
|
|
What proteins involved in the complement system circulate in the blood in their inactive forms?
|
C1-C9
|
|
|
What are the functions of the complement system?
|
1. Phagocytosis/ Destruction
-Opsonization -MAC 2. Vascular Inflammatory effects -Anaphylaxis= inc. vascular permeability = vasodilation 3. Cellular Inflammatory Effects -WBC Adhesion, activation, chemotaxis |
|
|
Describe the Complement System
|
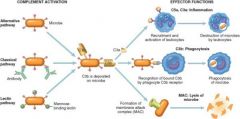
|
|
|
What are the Chemical mediators of Inflammation derived from plasma? (Hageman factor initiates what?)
|
Coagulation and Kinin Systems
Hageman Factor (Factor XII = 12) Initiates: "KCCF" Kinin System Coagulation Complement System Fibrinolytic System |
|
|
What is the Kinin Cascade?
|
Bradykinin --> Increased Vascular Permeability --> Arteriolar Dilation --> Bronchial Sm. M. Contraction (it also causes pain when injected into skin)
|
|
|
What are the cell-derived chemical mediators of inflammation?
|
1. Vasoactive Amines
2. Arachidonic Acid (AA) Metabolites (from phospholipids) 3. Platelet-Activating Factor (PAF) (from phospholipids) 4. Cytokines 5. Chemokines 6. Nitric Oxide (NO = Vasodilation) 7. Lysosomal Molecules -Acid and neutral proteases (=degrade cellular and ECM proteins) 8. Inferon (INF) 9.ROS 10. Neuropeptides (substance P = pain) |
|
|
What are AA Metabolites derived from?
|
Phospholipids via Phospholipases
Steroids inhibit Phospholipases = thus inhibiting AA Metabolites |
|
|
What is the function of the Lysosomal cell-derived chemical mediator of inflammation alpha-1-antitrypsin?
|
Inhibits PMN Elastase
Emphysema = Imbalance between protease and antiprotease results in alveolar wall destruction (=adjacent air spaces fuse together = very inefficient gas exchange = hyper-inflation of lungs) |
|
|
Review the table of cell mediators of inflammation
|

|
|
|
What are the Acute cells of inflammation?
|
Neutrophils (=PMNs)
Eosinophils Mast Cells |
|
|
What are the chronic cells of inflammation?
|
Lymphocytes
Macrophages Plasma Cells Eosinophils (Mast cells) Fibroblasts (prominet fibrosis) |
|
|
T/F: Basophils can become Mast cells
|
TRUE
|
|
|
T/F: Monocytes can become macrophages?
|
TRUE
|
|
|
T/F: Lymphocytes can become Plasma cells
|
TRUE
|
|
|
What are the 3 outcomes of the inflammatory process?
|
1. Resolution
2. Healing by Fibrosis 3. Ongoing Chronic Inflammation |
|
|
Gastric Ulcers result from chronic Acute Inflammation thanks to what bacteria?
|
Gastric Ulcers - Result from Chronic Acute inflammation process in stomach from H. pylori
|
|
|
What are the causes of Chronic Inflammation?
|
1. Persistent Infection or injurious stimulus
2. Prolonged exposure to injurious stimulus -can be Endogenous or Exogenous 3. Autoimmunity ex. = rheumatoid arthritis |
|
|
Describe the process of chronic inflammation
|
1. Tissue infiltration by mononuclear cells (macrophages, lymphocytes, plasma cells, Histocytes are long-standing resident in tissues
2. Tissue Destruction 3. Tissue Repair -Angiogenesis Fibrosis Typically results in Loss of Function |
|
|
Describe the wide variety of tasks that macrophages do in terms of 1. Inflammation and Tissue Injury and 2. Repair
|
1. Inflammation and Tissue Injury = ROS and Nitrogen species
Proteases Cytokines (including Chemokines) Coagulation Factors AA Metabolites 2. Repair Growth Factors Fibrogenic Cytokines Angiogenic Factors Remodeling (i.e. using collagen) |
|
|
Define Granulomatous Inflammation
|
A distinct pattern of chronic inflammation
Aggregates of activated macrophages assume epitheliod appearance (ie it looks like an epithelium cell) Some of the tissue coaleces to form a syncytium = multi-nucleated giant cell |
|
|
If Necrosis is present, what do we call granulomatous inflammation? If no necrosis is present, what do we call granulomatous inflammation?
|
Necrosis = caseating granuloma
NO Necrosis = Non-Caseating granuloma |
|
|
What are granulomas and what they do?
|
Granuloma = Mass of inflammatory cells
"Wall-off" offending agents (to contain them) |
|
|
What are some systemic effects of inflammation?
|
Fever
Elevated Plasma Acute Phase Reactants (for ex. C-reactive protein = CRP, Fibrinogen Leukocytosis (elevated circulating WBCs, such as PMNs = neutrophils and Lymphs) |
|
|
Describe the phenomenon of RBC Rouleaux and ESR
|
RBCs overall negative charge = they repel each other = they don't clump together.
Infection = Acute Phase Proteins = As their neg. charge decreases, the RBCs can start coming together Inc. Sed. Rate = Inc. Inflammatory Process |
|
|
Describe the difference between renewal and Repair
|
Renewal = Normal Physiological process
Repair = When there's damage = Regeneration = a parenchymal component |
|
|
Renewal and Repair begin with cell loss/injury- what are the responses to this?
|
Cell migration
Cell proliferation Deposition/Reorganization/Remodeling of extracellular matix components |
|
|
Give some examples of natural cell loss
|
Sloughed epithelia (skin, mucosa)
Balance of proliferation and apoptosis Mechanical Loss |
|
|
Give some examples of Pathologic cell loss
|
Injury/Inflammation destruction
Loss of Parenchymal cells Loss of stromal cells (i.e. mesenchymal, fibroblasts, etc.) that support function |
|
|
What function of natural cell loss maintains function?
|
Renewal
|
|
|
Are Regeneration and Fibrosis components of Renewal or Repair?
|
Repair
|
|
|
What are some processes involved in the repair of cells?
|
Regeneration of Cells
Healing - scar |
|
|
Whats involved in the maintenance of parenchymal cell function?
|
balance of proliferative activity where cells become mature and enter into specific roles.
Cells not needed = Apoptosis |
|
|
What regenerates cells in skin (hair + sweat glands), intestine, liver and corneal epithelium?
|
stem cells
|
|
|
In the liver, injury to ONLY hepatocytes is repaired how?
Injury to hepatocytes AND Matrix is repaired how? |
If ONLY Hepatocytes, repair = Regeneration
If Hepatocytes AND MATRIX = Laying down of fibrous tissue |
|
|
What types of fibers provide a scafolding in the liver for RBCs to percolate through and hepatocytes to attach to?
|
Reticular
|
|
|
Detail the process of repair in the liver if A. Only hepatocytes damaged B. Hepatocytes (parenchymal cells) and Scaffolding damaged
|
If only hepatocytes damaged, stem cells differentiate into hepatocytes
If Hepatocytes and scafolding damaged, stem cells don't have anything to attach to, so they form a regenerative nodule = there is a scar |
|
|
List the Cellular components of repair
|
1. Inflammatory Cells
2. Parenchymal Cells 3. Fibroblasts and other Stromal Cells --> Repair Tissue (structural integrity) 4. Endothelial Cells = Angiogenesis |
|
|
What are the two types of Fibrous Structural Proteins involved in the ECM?
|
Collagen
-Fibrillar: I (strong, late wound) II (Cartilage) III (early wound; hollow structures, V, IX -NonFibrillar = IV (basement membranes) Elastins -Adhesion Proteins (CAM, Cadherins, Integrins) -Proteoglycans and Hyaluronic Acid |
|
|
Collagen, a fibrous structural protein, is made up of what types?
|
Fibrillar:
I = Strong, late wound II = Cartilage (no wound-healing participation) III = Early wound, Loose CT = flexibility IV = Basement Membrane |
|
|
What is the role of p53?
|
Cell cycle regulation
Tumor Supressor = prevents genome mutation Can hold cell cycle at G1/S Can initiate Apoptosis |
|
|
In the healing of a wound, granulation tissue is eventually replaced by what tissue type?
|
collagen type I (=strong, late wound)
|
|
|
T/F: Superficial injury to the skin involves a lot of granulation tissue formation
|
FALSE. It involves minimal granulation tissue formation...primary goal = re-epithelialization
|
|
|
What is the overall goal of Healing and Fibrosis?
|
To maintain hemostasis = keep blood in blood vessels
|
|
|
List the steps in Healing and Fibrosis of wounds
|
Maintain Hemostasis
Inflammatory Response Proliferation and migration of Parenchyma Angiogenesis Synthesis and Deposition of ECM Tissue Remodeling Wound Contraction Acquisition of Wound Strength |
|
|
List the sequence of events in the wound healing process
|
1. Hemostasis
2. Inflammation 3. Provisional Matrix 4. Granulation Tissue 5. Fibroblast Proliferation and Collagen deposition/remodeling (=get rid of collagen III, replace with collagen I) 6. Re-epithelialization 7. Wound contraction 8. Inc. in wound strength |
|
|
What are the three overall phases of wound healing?
|
Inflammation
Proliferation Maturation |
|
|
Clot formation and chemotaxis occur in what stage of wound healing?
|
Inflammation
|
|
|
Granulation tissue involves lots of what type of collagen?
|
Collagen type I
|
|
|
Scar tissue involves lots of what type of collagen?
|
Collagen Type III
|
|
|
What are some conditions that can affect the repair of wounds during the healing process?
|
Location (ie ability of wound to contract less on scalp)
Amount and nature of ECM at Site of injury (older people have less collagen/ elastin) Blood Supply (dec. blood supply = dec. healing capacity) |
|
|
What are the local factors that can affect wound healing?
|
Infection
Mechanical Forces Foreign Bodies Size, Location, Type of Wound |
|
|
What are some Systemic factors that affect wound healing?
|
Nutritional status
Metabolic status Circulation Hormones |
|
|
What factors lead to cirrhosis of the liver?
|
Fibrosis with continued parenchymal and ECM damage
|
|
|
What are the key differences between primary skin wound healing and secondary skin wound healing?
|
Primary = neutrophils, clot, mitosis, macrophage, fibroblast, fibrous union
Secondary = Neutrophils, MANY new capillaries, wound contraction |
|
|
Whats the consequence of chronic hepatic injury?
|
Formation of regenerative nodules separated by fibrous bands
|
|
|
If the kidney is damaged, and injury does NOT significantly damage the ECM framework, what can we expect from the renal tubular epithelium? What if there is damage to the ECM framework?
|
epithelium will recover
Damage to ECM framework = scarring = we lose function |
|
|
T/F: Metaplastic processes are reversible
|
TRUE, as long as the stimulus is removed.
|
|
|
If the underlying stroma of the lung is damaged, what happens to the tissue?
|
Fibrosis = impairs gas exchange
|
|
|
Loss of cardiac myocytes results in what?
|
Fibrosis
|
|
|
Diffuse loss of cells in the heart leads to what?
|
Myocardial Infarction, following Coagulative Necrosis
|
|
|
T/F: The heart can only replace damaged/lost cells with a scar
|
TRUE
|
|
|
In Lung, what are our two options in terms of repair?
|
If damage limited to pneumocytes, that's OK bc they'll divide/replicate
I damage to Alveolar epi AND scaffold/matrix = thickened fibrosis - inefficient respiration |
|
|
T/F: Axons in PNS can elongate/grow and remake synapses, while CNS axons cannot
|
TRUE
|
|
|
How is scarring accomplished by neurons?
|
PNS = Fibrosis
CNS = Glial cell proliferation |
|
|
What are some problems with excessive wound healing?
|
1. Excessive scar formation
2. Keloid 3. Excessive Contraction = Contracture (palms, soles) |
|
|
What is a Keloid?
|
Over-deposition of collagen within dermis = the keloid extends beyond the anatomic confines of normal structure
|
|
|
What type of excessive wound healing problem is common when someone is burned?
|
excessive contraction = Contracture = deformity of wounded tissue and surrounding structures
For ex., when people burn their hands, excessive contracture can make them have to hold their hands inwards, with wrist bent in. |
|
|
What are some examples of Excessive Regeneration/Repair that can occur during wound healing?
|
Excessive Granulation tissue formation
-Pyogenic Granuloma -Proud Flesh Excessive Fibrosis after injury -Desmoids |
|
|
What force within blood vessels allows the vessel to hold onto or maintain fluid?
|
Plasma colloid osmotic pressure
|
|
|
What force within blood vessels allows the vessel to lose blood?
|
Hydrostatic Pressure
|
|
|
The primary insult in a hemodynamic disorder has to do with what factors?
|
Tissue injury and too much bleeding (or too much clotting)
|
|
|
A secondary insult in a hemodynamic disorder has to do with what?
|
secondary hemodynamic disorder = something in body is affecting blood vessels
examples = congestive heart failure, shock, liver disease (chronic alcohol abuse) |
|
|
What is Edema?
|
excessive fluid accumulation in spaces outside blood vessels.
Know some causes of edema |
|
|
What are 3 major causes of systemic edema?
|
Heart Failure
Mal-Nutrition (remember mal-nourished kids in DR with edema...) Anything leading to decreased Synth. of albumin by liver, i.e. nephrotic syndrome = lose a lot of protein in urine |
|
|
How does decreased plasma albumin lead to edema?
|
Dec. plasma albumin = Dec. Oncotic Pressure = Edema
OR Inc. Hydrostatic pressure = Edema (directly) (for. ex. Inc. Na+) Heart Failure = Inc. Capillary Hydrostatic Pressure = Dec. Blood flow (Inc. blood vol.) = Edema |
|
|
Whats the difference between dependent edema and pulmonary edema?
|
Dependent edema = gravitational influences on interstitial edema (RV failure predominates)
Pulmonary Edema = Congestion (LV failure predominates) |
|
|
Whats the difference between Generalized edema and Cerebral Edema?
|
Generalized = renal dysfunction, nephrotic syndrome
Cerebral Edema = Localized; infection neoplasm, stroke -Vasogenic = vessel damage -Cytotoxic = Intracellular -Interstitial = Extracellular |
|
|
Whats the difference between Hyperemia and Congestion?
|
Hyperemia = Active, Blood flow INTO tissue
Congestion = Passive, Blood flow OUT of tissue |
|
|
Define Hyperemia
|
Active increase of blood INTO a tissue
|
|
|
Define Congestion
|
Passive, impaired out-flow of blood OUT of a tissue (cOngestion - Out)
|
|
|
What are the 4 types of Hemorrhage?
|
"HEPP"
Hematoma = large collection of blood in tissue Ecchymosis = Bruises Petechiae = Pinpoint hemorrhages Purpura = Red/purple skin discoloration |
|
|
Between a Petechiae hemorrhage and a Hematoma Hemorrhage, which causes the least amount of vessel damage?
|
Petechiae (bc its pinpoint hemorrhages instead of a massive large hemorrhage)
|
|
|
Is hemostasis a pathological or physiological process?
|
Physiological
|
|
|
Are thrombosis and bleeding pathological or physiological processes?
|
Pathological
|
|
|
What are the steps of hemostasis when a injury to the skin occurs?
|
1. Vasoconstriction
2. Primary Hemostasis = platelet plug (subendothelial ECM, vW Factor) 3. Secondary Hemostasis 4. Localized Thrombus Formation |
|
|
T/F: At the same time coagulation is going on, anti-coagulation is going on to keep coagulation localized
|
TRUE
|
|
|
What happens to the clotting process if we don't have von Willebrand Factor (Factor XII, 12)?
|
Adhesion is negatively affected = cells don't adhere = no clotting
|
|
|
What are some Pro-Thrombotic vs. Anti-Thrombotic Factors?
|
Pro = platelets, Coagulation, Anti-fifrinolytic (=eats frinolytic=more fibrin= pro-clotting)
Anti = Intact Endothelium, Anti-Caogulation, TFP1, Fibrinolytic |
|
|
What does Protein C do in the process of inhibiting thrombosis?
|
Proeolysis of factors 5a (Va) and 8a (VIIIa)
Requires Protein S |
|
|
What does a deficiency of Protein C or Protein S mean in terms of clotting?
|
Dec. Protein C or Protein S = Over-Clotting
|
|
|
Coagulation requires what two substrates to make the assembly substrate?
|
Calcium and Phospholipid
|
|
|
How do we test for the presence or absence (or just general amount) of clotting factors in the blood?
|
Give a Ca2+ kelator = Ca2+ removed = Blood inhibited from clotting = we can test presence of clotting factors that are free flowing in blood
|
|
|
What are 3 "natural" anticoagulants?
|
Anti-thrombins
Protein C and Protein S TFPI |
|
|
T/F: The Extrinsic and Intrinsic pathways lead to the common pathway in the coagulation cascade
|
Yep.
|
|
|
The Intrinsic pathway of coagulation is made up of what types of factors? (contact or tissue?)
|
Contact factors
|
|
|
The Extrinsic pathway of coagulation is made up of what types of factors? (contact or tissue?)
|
Tissue Factors
|
|
|
The bulk of lab testing of the Coagulation Cascade is performed in:
A. Primary Hemostasis B. Secondary Hemostasis C. Fibrinolysis |
B, Secondary Hemostasis
-Pro-thrombin Time (PT), aPTT (activated partial thromboplastin time), TT (Thrombin Time |
|
|
Whats the path of Fibrinolysis?
|
Plasminogen --> Plasmin --> cleaves Fibrin --> which leads to Fibrinolysis (one FSP = D-Dimer)
|
|
|
D-Dimer measurement in the lab is important in to tell us what?
|
How much blood clot break down is occurring
|
|
|
Thrombosis is a Pathological process because of what?
|
Endothelial Injury (*most significant)
Stasis Hypercoagulable State |
|
|
What is the most significant factor that promotes thrombosis?
|
Endothelial Injury
|
|
|
What are the causes of Endothelial Injury?
|
Hypertension
Turbulent Flow Inflammation |
|
|
T/F: Congestion and Turbulence, which disrupt normal laminar flow through vessels, can lead thrombosis
|
TRUE
|
|
|
How does Primary Thrombosis differ from Secondary Thrombosis?
|
Primary Thrombosis = Genetic
Secondary Thrombosis = Acquired (secondary to other disease conditions) |
|
|
What are three Primary Hypercoaguable States?
|
1. Factor V (Leiden) gene mutation
2. Prothrombin Gene Mutation 3. Hyperhomocysteinemia |
|
|
What is a Factor V (Leiden) Gene mutation?
|
Factor V is resistant to cleavage by Protein C = can't inactivate Factor 5 - its active and promotes clotting
|
|
|
What is a Prothrombin gene mutation?
|
Excessive amounts of Pro-thrombin = Hypercoaguable
|
|
|
What is Hyperhomocysteinemia?
|
Inhibition of Anti-Thrombin III and Thrombomodulin (can be acquired OR genetic)
|
|
|
Name some examples of Acquired Hypercoaguable states
|
Things associated with stasis or vascular injury = heart failure, MI, atherosclerosis, inactivity
Hepatic (Inc. Synth. of Clotting Factors, Dec. Synth. of Anti-Thrombin III) Cancers Heparin-induced Ab's that activate platelets Obesity, Smoking. SO DON'T DO IT! Antiphospholipid Syndrome |
|
|
What are the fates of a thrombus, once formed?
|
Dissolution
Propagation Organization/Recanalization Embolization |
|
|
What is DIC, Disseminated Intravascular Coagulation
|
Widespread thrombin activation with production of fibrin thrombi with microvascularture
Can result ultimately in increased bleeding (=platelet count decreases. More prominent in brain, kidney, heart, lungs, liver) |
|
|
What is an embolism?
|
Dislodged or detached portion of solid, liquid or gas that travels through blood vessels (=Hypoxia and Ischemia)
WHERE Thrombi is determines which circulatory system affected (pulmonary or systemic) and likelihood of impairing blood flow to certain organs |
|
|
What are the dif types of embolisms?
|
Fat
Air/Gas Tumor Blood Clot (Thromboembolism) |
|
|
Pulmonary Embolisms are most frequently derived from what?
|
DVT's
|
|
|
What percent of cardiac thrombi are derived from cardiac mural thrombi?
|
80%
Most from LV MI |
|
|
What percent of infarcts are due to Thromboembolic events?
|
95% (most occur in heart and brain)
|
|
|
What are the different types of Infarct?
|
Hemorrhagic = Red = Venous Occlusion
Anemic = White = Arterial Occlusion |
|
|
Most Infarcts (except the brain) include what type of tissue cell death?
|
Ischemic Coagulative Necrosis
(EXCEPTION = BRAIN = LIQUEFACTIVE NECROSIS) |
|
|
Development of an infarct is dependent on what?
|
Rate of vascular decline (if infarct is not severe, damage will occur more slowly)
|
|
|
What are the relative times the brain, heart, and fibroblasts are vulnerable to hypoxia?
|
Brain = 3-4 min
Heart = 20-30 min Firbroblasts = Many hours |
|
|
What is Shock?
|
Cardiovascular collapse with global hyperfusion of tissues and subsequent anoxia/ischemia
Microvascular Thrombosis Vasodilation = inc. permeability = dec. perfusion Immunosuppression |
|
|
What are the major causes of shock?
|
Cardiogenic (heart failure)
Hypovolemic Septic Neurogenic Anaphylactic |
|
|
What causes Cardiogenic Shock
|
MI
Cardiac Arrhythmias Pulmonary embolism Cardiac Temponade |
|
|
What is Hypovolemic shock?
|
Reduction of fluid volume some way or another
Things than can cause it: Burns Severe Dehydration (diarrhea, vomiting, excessive perspiration) Lack of fluid intake to restore blood volume |
|
|
What is Septic Shock?
|
Bacterial Infections
Gram Negative Septicemia; bacteria cause vasodilation and hypotension Disseminated Intravascular Coagulation (DIC) Multiple End-Organ Failure "Shock Lung" Mortality of Septic Shock = 25-50% |
|
|
What are some causes of Neurogenic Shock?
|
Anesthetic States
Brain/Spinal Cord injury |
|
|
What is Anaphylactic Shock?
|
General Vasodilation from Type I Hypersensitivity Reactions
|
|
|
What are the Stages of Shock?
|
Stage I = Compensation (inc. sym. tone)
Stage II = Decompensation (dec. tissue perfusion) Stage III = Irreversible (necrosis and organ failure) |
|
|
What is Thrombocytopenia?
|
Decreased Platelets = spontaneous bleeding (reduced clotting)
Normal PT and PTT Increased Destruction of TTP (thrombocytopenic Purpura) |
|
|
What is Von Willebrand Disease?
|
Decreased amount of vWF
Spontaneous bleeding through mucous membranes Normal PT, may see prolonged PTT |
|
|
What are some clinical bleeding Caogulation disorders?
|
Hemophilia A
Hemophilia B Acquired Bleeding Disorders (Vit. K deficiency, Liver disease) |
|
|
What is Clinical Antiphospholipid Syndrome (APS)?
|
Ab's directed against phospholipids
|
|
|
Developmental Abnormalities typically result in A. Congenital B. Hereditary/Familial Disorders
|
A. Congenital
|
|
|
What are the 3 classifications of genetic disorders?
|
1. Chromosomal
2. Disorders related to single genes with pronounced effects 3. Complex multigenic disorders |
|
|
Whats the most common genetic abnormality?
|
Multigenic (=variable contribution of many dif. alleles)
|
|
|
T/F: Of the known causes of congenital abnormalities, the majority are heritable in cause
|
TRUE
|
|
|
Define Angenesis
|
Absence of organ or anlage (Anlage = initial clustering of embryonic cells from which organ develops)
|
|
|
Define Aplasia
|
Persistence of Anlage or rudiment w/o mature organ
|
|
|
Define Hypoplasia
|
Reduced size from incomplete development
|
|
|
Define Dysraphic Anomaly
|
Failure of opposed structures to fuse
|
|
|
Define Involution Failure
|
Inappropriate persistence of structures beyond developmental stage
|
|
|
Define Division Failure
|
Incomplete cleavage of embryo
|
|
|
Define Atresia
|
Incomplete formation of lumen or tubular structure
|
|
|
Define Dysplasia
|
Abnormal histogenesis
|
|
|
Define Ectopia/ Heterotopia
|
Organ/Tissue situated outside normal location
|
|
|
Define Dystopia
|
Inadequate migration of organ, which remains in developmental location
|
|
|
Teratogenic exposure In-Utero has the most significant deleterious effects before what time point?
|
16 weeks
Week 3--> Week 16 = teratogens have major morphogenic affects |
|
|
What are the signs/symptoms of FAS, Fetal Alcohol Syndrome?
|
Small Head (Microcephaly)
Epicanthal folds (=skin fold of upper eyelid) Short Palepbral Fissure (separation between upper+lower eyelid) Maxillary Hypoplasia Thin Upper Lip Poorly formed Philtrum Cardiac Septal Defects Cognitive/Behavior effects |
|
|
What are some Infectious Biological Teratogens? (Teratogen = interferes with embryo development)
|
"TORCH"
Toxoplasma Other (Syphilis, Varicella-zoster, EBV, TB) Rubella CMV Herpesvirus (HSV 2) |
|
|
What are some of the signs/symptoms if a baby is exposed to some of the infectious biological teratogens (TORCH)?
|
small head
calcifications present small, closely-set eyes petichiea purpura (scattered over skin/face) |
|
|
What do cytogenics tell us?
|
Abnormalities in chromosome number and/or structure
|
|
|
When do chromosomal abnormalities arise?
|
Somatic cell division (mitosis) (Abnormalities in chromo # varies by cell)
OR Gametogenesis (if chromo # changed here, all cells will have same # chromos) |
|
|
What are some abnormal chromosome structures?
|
Translocations
Deletions Inversions |
|
|
What is balanced reciprocal Translocation of chromosomes?
|
When chromsomes mix equally (as opposed to Robertsonian centric fusion, when chromos mix unequally)
|
|
|
What is Robertsonian centric fusion of chromosomes?
|
When chromozomes mix unequally, for ex. there is one small chromo and one huge chromo created
|
|
|
What is Trisomy 21?
|
Downs Syndrome
|
|
|
What is Trisomy 13?
|
Patau's Syndrome
|
|
|
What is Trisomy 18?
|
Edward's Syndrome
|
|
|
What's the most common cause of congenital (=at birth) retardation?
|
Trisomy 21, Down's Syndrome
|
|
|
T/F: Aberrations of sex chromosome number typically produce less severe results than abnormal numbers of autosomes
|
TRUE
Ex. = Klinefelter Syndrome (testicular diagenesis) |
|
|
What is Turner Syndrome?
|
Complete or partial loss of one X chromosome
-Sexual infantilism (poor development) Neck "wings" out Possible genetics: 45, X |
|
|
What are some of the single gene mutations?
|
Point mutations
Frameshift mutations Trinucleotide Repeat |
|
|
What are some examples of Mendelian Inheritance of Genetic Mutations
|
Autosomal Dominant
Autosomal Recessive Sex-linked Dominant Sex-Linked Recessive Codominance Importance of Penetrance and Expressivity |
|
|
T/F: Most Autosomal Dominant genetic mutations are expressed in a heterozygous state
|
TRUE
|
|
|
What is Ehler's Danlos-Syndrome?
|
Connective tissue autosomal dominant disorder
|
|
|
What is the difference between NF1 and NF2, both Neurofibromatosis diseases?
|
NF1 = Von Recklinhausen disease. Chromosome 17. Dark skin pigmentation (cafe-au-lait spots...think of woman at festival...)
NF2 = Central neurofibromatosis. Chromosome 22. Tumors of Schwann Cells |
|
|
Whats the most common and lethal Autosomal Recessive genetic disorder?
|
Cystic Fibrosis (1 in 25 caucasians)
Chloride channel mutation = thick mucous Cl- moves IN cell = Na+ moves IN cell = water follows Na+ = mucous dehydrated = mucous thick = Clogged pancreatic duct = pancreatitis = improper # digestive enzymes = No lipases = can't digest fat = fatty diarrhea (statorhea) |
|
|
What is Lysosomal Storage Disease?
|
Defect in Lysosomal Hydrolases
Ex. = Gaucher Disease (deficiency in glucosylcerebrosidase) |
|
|
What is Tay-Sach's Disease?
|
Ashkenazi Jews
GM2 ganglioside accumulates in lysosomes, (neurons of brain and cells of retina) Progressive motor, cognitive deterioration |
|
|
What chromosome does Cystic Fibrosis affect?
|
7q
|
|
|
What chromosome does Sickle Cell Anemia affect?
|
11p
|
|
|
What chromosome does Gaucher disease affect?
|
1q
|
|
|
What chromosome does Tay-Sachs affect?
|
15q
|
|
|
What is Fragile X Syndrome?
|
Most Common form of INHERITED (not congenital, which is Down's Syndrome) Mental Retardation
|
|
|
List some X-linked Recessive Disorders
|
Fragile X Syndrome
Hemophilia A (Factor VIII deficiency) Duchenne-Becker Muscular Dystrophy |
|
|
What is a trinucleotide repeat disorder?
|
Same 3 Nucleotides repeat over and over
The number of repeated segments often increases with successive generations |
|
|
What are some examples of trinucleotide repeat diseases?
|
Huntington Disease
Fragile X Syndrome |
|
|
What are some examples of X-linked dominant Diseases?
|
Vitamin D-resistant Rickets
Alport's Syndrome |
|
|
What type of genetic disorders are probably the most common cause of genetic disease?
|
Complex Multigenic Disorders
Ex. = Atherosclerosis and Diabetes |

