![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
429 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
T/F: Lymphocytes, Phagocytes and Mediator cells are all derived from hematopoietic stem cells
|
TRUE
|
|
|
|
Whats the difference between primary lymphoid organs and secondary lymphoid organs?
|
Primary = where immune cells ARISE (bone marrow, thymus)
Secondary = where immune cells come together to initiate adaptive immunity (lymph node, spleen) |
|
|
|
What are the three major functions of the immune system?
|
Defense, Homeostasis, Surveillance
|
|
|
|
Define Defense, a function of the immune system
|
prevent disease caused by microbes, viruses, parasites, fungi, bacteria
|
|
|
|
Define Homeostasis, a function of the immune system
|
Turn off immune responses to prevent over-reaction - remove senescent cells (for ex. red cells)
|
|
|
|
Define Surveillance, a function of the immune system
|
Seek out and destroy altered self (for ex. malignant cells)
|
|
|
|
T/F: The immune system can cause disease
|
TRUE.
It can act as barrier to stem cell therapy, transplantation and gene therapy Innapropriate responses cause disease (immune-related inflammatory diseases) |
|
|
|
Which of the following are immune-related diseases?
Allergy, Autoimmunity, Inflammatory bowel disease, Type 1 Diabetes, Celiac Disease, Transplantation Rejection |
ALL
|
|
|
|
Define what is meant by opportunistic pathogen
|
A microbe that is a part of our normal flora that causes disease
|
|
|
|
List the major categories of pathogens
|
Viruses
Fungi Bacteria Protozoan Parasites Helminth Parasites |
|
|
|
T/F: Bacteria are eukaryotes
|
FALSE, they are prokaryotes, son.
|
|
|
|
What are the to shapes bacteria can take?
|
Bacilli (rods)
Cocci (Spherical) |
|
|
|
What does it mean for a bacteria to be Gram +?
|
Its has peptidoglycan on membrane surface
|
|
|
|
What doe sit mean for a bacteria to be Gram -?
|
It has Lipopolysaccharide and Peptidoglycan on membrane surface
|
|
|
|
Viruses, obligate intracellular parasites, can be made from DNA OR RNA
|
YES
|
|
|
|
What are the shapes of viral capsids?
|
Icosahedral or Helical, with or without a lipid envelope
|
|
|
|
Define Protozoa
|
single-celled eukaryotes, transmitted by insect vectors
|
|
|
|
What are Helminths?
|
Parasitic worms that are highly differentiated multi-cellular eukaryotes with complex life cycles
|
|
|
|
T/F: Fungi are eukaryotes
|
TRUE
|
|
|
|
What are the shapes of fungi?
|
Round yeast cells
Filamentous Hyphae |
|
|
|
What are the major contents of fungi cell walls and membranes?
|
Cell Wall: Chitin
Cell Membrane: Ergosterol |
|
|
|
What is the main way the immune system is able to defend against things like fungi, helminths, microbes, bacteria, and protozoa?
|
Its ability to differentiate between self and non-self, because microbes have molecules that are different from ours (= called Pathogen-Associated Molecular Patterns, PAMPs)
|
|
|
|
Which of the following are considered "non-self" to the immune system? Flagella, Cancer Cells, Lymphocytes, Virus-infected cells, Senescent cells, Transplanted/mismatched cells, yeast, Muscle cells
|
Everything on list except Lymphocytes and Muscle Cells
|
|
|
|
Describe the differences between a Gram + cell wall and a Gram - cell wall
|
Gram +: cell wall contains ONLY Peptidoglycan, a PAMP (pathogen-associated molecular pattern), on top of a plasma membrane
Gram -: Contains Peptidoglycan, then an outer cell membrane, then Lipopolysaccharide (also a PAMP) |
|
|
|
What are the two "arms" of the immune response?
|
Innate Immunity
Adaptive Immunity Both have ways to differentiate between self and non-self |
|
|
|
T/F: Innate immunity, the first response to a pathogen, is usually sufficient to resolve the majority of infections (i.e. no perceptible disease occurs)
|
TRUE
|
|
|
|
What are the characteristics of Innate Immunity?
|
First response to a pathogen
Usually sufficient to resolve the majority of infections Fast, always present Non-specific (=can recognize common elements of bacteria, viruses etc. like PAMPs) No Memory (=won't recognize pathogen if it sees it a second time= when it sees it again it responds the same as the first time) Limited types of responses that are non-specific for a given microbe (for ex, it recognizes peptidoglycan of all Gram+ bacteria) |
|
|
|
T/F: Innate immunity stimulates the development of adaptive immunity
|
TRUE
|
|
|
|
Describe the characteristics of Adaptive immunity
|
Set in motion by innate immunity
Second response to a pathogen Slower response than innate immunity b/c its tailored/adapted to deal with specific features of pathogens Has a Memory (when it sees pathogen a second time, it remembers it and mounts a faster, stronger and more specific response) |
|
|
|
T/F: The Recognition mechanisms for Innate immunity are variable.
|
FALSE. The recognition mechanisms of innate immunity are Fixed, i.e. the same response occurs every time it comes across pathogen (Adaptive immunity is variable.)
|
|
|
|
Whats the difference in time frames for Innate and Adaptive Immunity?
|
Innate = hours (fast)
Adaptive = days to weeks(slow) |
|
|
|
T/F: The innate response is constant during the duration of the response
|
TRUE
|
|
|
|
T/F: Inflammation is a part of the Adaptive immune response
|
FALSE. Inflammation is a part of the INNATE immune response
|
|
|
|
What are the general steps involved in an Adaptive Immune Response?
|
Pathogen (say bacteria) enters via skin
Dendritic cell travels via lymphatics to Lymph node where it stimulates B cells = Abs against bacteria B cells travel in blood back to site of infection |
|
|
|
T/F: Neutrophils and Macrophages are only involved in Innate immunity
|
FALSE. They are involved in both innate and adaptive immunity
|
|
|
|
What component of the immune system causes an initial rash upon infection with a pathogen?
|
Innate
|
|
|
|
When a pathogen infects a host, what component of the immune response disallows it from causing more severe illness than what it initially causes?
|
Adaptive Immune Response
If the immune system fails to eliminate pathogen, the disease progresses to a more chronic form |
|
|
|
T cells and B cells are what types of cells?
|
Lymphocytes
|
|
|
|
List some common phagocytes
|
Monocytes, Macrophages, Dendritic cells, Neutrophils
|
|
|
|
List some common mediator cells
|
Basophils, Eosinophils, Natural Killer cells, Mast cells
|
|
|
|
Where do B cells develop?
|
Bone Marrow (B=Bone Marrow)
Naive B cells capture antigen and become plasma cells that make antibiotics |
|
|
|
Where do T cells, which coordinate the entire immune response, develop?
|
Thymus (T=Thymus)
|
|
|
|
Naive T cells are activated by what?
|
Antigen-presenting cells, then they become Effector T cells (because they exert an Effect!)
|
|
|
|
T/F: T cells can help B cells become what they need to be by secreting cytokines
|
TRUE
T cells help B cells undergo clonal expansion and differentiation into Ab-secreting plasma cells |
|
|
|
T/F: Helper T cells (Th) are CD4+ and help B cells do their job
|
TRUE (T-Helper cell = 4 syllables = CD4+)
|
|
|
|
Define cytokines
|
Produced by T Helper cells.
They are proteins that affect the behavior of other cells |
|
|
|
What are some of the things T Helper cells do?
|
Help B cells become plasma cells
Enhance Macrophage function |
|
|
|
T/F: Interleukin is a type of cytokine
|
TRUE
|
|
|
|
Define Interleukin
|
A generic name for a cytokine produced by leukocytes
("cytokine" is a more general term) |
|
|
|
Define Cytotoxic, and list a cell that is cytotoxic
|
The killing of host cells infected with intracellular pathogens (for ex. viruses)
Cytotoxic T Lymphocytes (CTL or Tc) |
|
|
|
T/F: CTLs, or Cytotoxic T Lymphocytes, are CD8+
|
TRUE (Cy-to-tox-ic-T-lymph-o-cyte = 8 syllables)
(vs. T Helper cells, which are CD4+) |
|
|
|
What cells are the effectors of Adaptive Immunity?
|
B and T cells
|
|
|
|
What are the two different types of Adaptive Immunity?
|
Humoral and Cellular
|
|
|
|
Define Humoral Immunity, a type of Adaptive immunity
|
Mediated by B cells and Antibodies, effective against extracellular microbes
|
|
|
|
Define Cellular Immunity, a type of Adaptive Immunity (AKA cell-mediated immunity)
|
Mediated by T cells
Effective against intracellular pathogens, including microbes that are ingested by macrophages and viruses that infect cells) Cellular Immunity = T cells = Intracellular Pathogens (C-T's look INside your body = INtracellular pathogens) |
|
|
|
Define Antigen
|
Any molecule that can bind specifically to an Ab or generate peptide fragments that can be recognized by the T cell's receptor
|
|
|
|
What do phagocytes do?
|
Seek out/take up microbes or microbial antigens by a process termed phagocytosis (hence the cell name)
|
|
|
|
T/F: Phagocytes can function as antigen-presenting cells
|
TRUE
Capture Antigens and present them to T Lymphocytes in lymph node |
|
|
|
What are the 4 classes of phagocytes?
|
"MMDN"
Macrophages Monocytes (take things up and KILL!) Dendritic Cells(most potent antigen-presenting cell) Neutrophils (take things up and KILL!) |
|
|
|
Where do Macrophages and Dendritic cells go after they take up antigen?
|
Lymph Node
|
|
|
|
What do Dendritic cells do?
|
Circulate in tissue/blood and capture microbes and antigens then go to lymph node
|
|
|
|
T/F: Neutrophils (=Polymorphonuclear Leukocytes, or PMNs) present antigen in lymph node
|
FALSE.
They engulf/kill extracellular pathogens. They are NOT antigen presenting cells |
|
|
|
List the mediator cells
|
"BEN M."
Basophil Eosinophil Natural Killer Cells (NK Cells) Mast Cells |
|
|
|
What do Basophils, Eosinophils and Mast Cells do?
|
Granulocytes that contribute to allergic responses and play a role in eliminating parasites
|
|
|
|
What do Natural Killer Cells (NK Cells) do?
|
Kill Virus-infected cells AND certain tumor cells
|
|
|
|
What of the following cells participate in the ADAPTIVE immune response?
B Cells Eosinophils T Cells Macrophages Mast cells Neutrophils Dendritic Cells |
B Cells
T Cells Macrophages Dendritic Cells |
|
|
|
Which of the following are antigen-presenting cells?
T Cells Eosinophils Dendritic cells Macrophages Neutrophils NK Cells |
Dendritic cells
Macrophages |
|
|
|
Where do immune cells live?
|
Lymphoid Tissue
Primary: Bone Marrow and Thymus Secondary: Tonsils, Spleen, Scattered Lymph Nodes, Mucosa-Associated Lymph Tissue |
|
|
|
T/F: Lymphocytes stay in the lymph and only exit when called to do so by immune system
|
FALSE. Lymphocytes circulate continuously between blood and lymph
Cells, microbes and antigens can enter lymph nodes from the blood OR the lymph |
|
|
|
Define Germinal Center
|
Site of INTENSE B Cell Proliferation (where B cells are activated)
|
|
|
|
Where is adaptive immunity induced?
|
Lymph Node
|
|
|
|
Where are B cells located in Lymph Node? T cells?
|
B Cells = Lymphoid follicle (outer edge)
T Cells = Right inside lymphoid follicle ("B Cells are on the Barrier") |
|
|
|
Where are T cells located in the Thymus?
|
Cortex
T cells move towards the medulla as they mature |
|
|
|
What percentage of T cells fail to develop or display self-reactivity, and thus are programmed to die (=called central tolerance)?
|
95%. This is called central tolerance
|
|
|
|
Where do Scenescent RBCs go to die?
|
Red pulp of spleen, which filters blood
("RBC's go to Red pulp to die") |
|
|
|
What is contained in the white pulp of the spleen?
|
B cells and T cells (Spleen has aggregations of lymphocytes similar to those in lymph nodes)
|
|
|
|
How do cells and microbes enter and leave the spleen?
|
Blood, as opposed to using blood AND lymph like in lymph system (makes sense, since spleen filters blood, and is a secondary lymphoid organ where adaptive immune responses are initiated!)
|
|
|
|
What constitutes our normal "flora" (AKA commensal flora)
|
Microorganisms (bacteria+fungi)
|
|
|
|
Define Pseudomembraneous Colitis
|
Infection of Large Intestine with over-growth of bacteria
|
|
|
|
T/F: Some of our normal flora in gut produce antimicrobials that inhibit the growth of pathogens
|
TRUE
|
|
|
|
List some of the barriers in the intestine to infection
|
bacterial flora, mucus, glycocalyx, M cell, Paneth cells (secrete defensins), Innate immune cells in Peyer's Patches, Epithelial cells (with tight junctions), Glycocalyx (-thick sugar layer)
|
|
|
|
What are Defensins?
|
Anti-microbial peptides that penetrate microbial membranes, disrupting membrane
Produced by Paneth Cells (and neutrophils, epi cells) |
|
|
|
Where do Defensins work in the body?
|
Intestine, Placenta, Stomach, Eyes, Oral Cavity, Urogenital Tract
|
|
|
|
What protects the stem cells of the crypts in the gut?
|
Paneth Cells
|
|
|
|
T/F: Receptors of the innate immune response don't change
|
TRUE
|
|
|
|
What are the outcomes of the innate immune response?
|
Inflammation (via cytokine and chemokine production)
Phagocytosis (via macrophages/neutrophils) Cell-mediated Apoptosis (NK Cells) Initiation of Adaptive Immunity |
|
|
|
List some major PAMPs of different organisms
|
Viruses: ssRNA, dsRNA, unmethylated DNA
Bacteria: Peptidoglycan, Glycolipids, Lipoproteins, Flagellin, Inmethylated CpG Nucleotides Gram+ Bacteria: Lipoteichoic Acid Gram- Bacteria: Lipopolysaccharides Fungi: Zymosan Protozoa: Glycophosphatidylinositol |
|
|
|
What are Toll-Like Receptors, and what do they recognize?
|
TLR3: detects dsRNA (viruses)
TLR4: Lipopolysaccharides (Gram- bacteria) TLR5: Flagellin (motile bacteria) TLR7: ssRNA (viruses) TLR8: ssRNA (viruses) TLR9: Unmethylated CpG DNA (Bacteria) Viruses = TLR 3, 7, 8 Bacteria = TLR 4, 5, 9 |
|
|
|
T/F: PAMPs are not expressed by our tissues or cells
|
TRUE
|
|
|
|
T/F: PAMPs are not exclusive to pathogenic microorganisms but are also part of our commensal organisms
|
TRUE
|
|
|
|
When a TLR recognizes a PAMP, whats its signaling mechanism?
|
NF kappa B (an important transcription factor) = Drives inflammatory cytokine production
TLR recognizes bacteria - MyD88 - NF kappa B/Ikappa B - Pro-inflammatory Cytokines produced |
|
|
|
What is the function of Myeloperoxidases?
|
Catalyze the conversion of H2O2 to Hypohalous Acid = Amplifies toxicity of ROS generated during respiratory burst
|
|
|
|
Neutrophils require what to kill bacertia and fungi?
|
Respiratory burst
NADPH Oxidase converts NADPH +O2 to NADPH + O2 + H= |
|
|
|
What does Superoxide Dismutase do?
|
Converts H+ and O2 to Hydrogen Peroxide (H2O2) and O2.
Reverse rxm catalyzed by catalase |
|
|
|
Define Chronic Garnulomatous Disease
|
Defect in NADPH Oxidase System
Immunodeficciency caused by defects in NADPH Oxidase System of enzymes that generate the superoxide radical involved in bacterial killing |
|
|
|
Persistent bacterial infections are commonly associated with what cellular defect?
|
Phagocytic cellular defects
|
|
|
|
Leukocyte Adhesion Deficiency causes what?
|
Widespread Pyogenic Bacterial Infection
|
|
|
|
Chronic Granulomatous Disease causes what?
|
Intracellular and Extracellular Infection, Granulomas
|
|
|
|
G6PD (Glucose-6-Phosphate) Deficiency causes what?
|
Defective Respiratory Burst, Chronic Infection
|
|
|
|
Myeloperoxidase Deficiency causes what?
|
Defective Intracellular Killing, Chronic Infection
|
|
|
|
Chediak-Higashi Syndrome causes what?
|
Intracellular and Extracellular Infection, Granulomas
|
|
|
|
Whats the role of Myeloperoxidase?
|
Converts H2O2 to Hypohalvous Acid
|
|
|
|
What is required for Innate Immune cell homing to sites of infection?
|
1. Innate immune cell interaction with vascular endothelium = allows cell to leave bloodstream to enter tissue
2. Chemokines (chemotactic cytokines) = Induce directed migration of cells to sites of inflammation/infection |
|
|
|
What molecules direct Neutrophils to sites of infection
|
Adhesion Molecules
Selectin-mediated adhesion is weak, and allows the neutrophils to roll along the endothelial walls of vessels |
|
|
|
List the steps involved in neutrophil extravasation (=neutrophil leaving blood vessel)
|
1. Rolling Adhesion (via Selectin binding to glycoproteins)
2. Tight Binding (via LFA-1, which neutrophil up-regulates. ICAM-1, Intercellular Adhesion Molecule-1, helps too) 3. Diapedesis ("Ped" = Foot. Neutrophil makes a foot to squeexe through membrane) 4. Migration |
|
|
|
T/F: Chemokine CXCL8 recruits Neutrophils to sites of infection
|
TRUE
|
|
|
|
How do NK cells (Natural Killer Cells) Distinguish between healthy and unhealthy cells?
|
They have an Inhibitory Receptor and an Activating Receptor
Inhibitory Receptor Recognizes "self" = prevents NK killing of host cell Activating receptor recognizes stress signals = promotes NK killing of target cell |
|
|
|
How do NK cells recognize "self" so as not to kill host cell?
|
Inhibitory Receptor recognized MHC class I, which is expressed by ALL nucleated cells
MHC1 sends neg. signal to NK cell not to kill |
|
|
|
T/F: Natural Killer Cell Activating Receptors can detect stressed cells
|
TRUE
Stressed cells express unique cell surface proteins that are detected by activating receptors |
|
|
|
T/F: Stressed host cells of a virus will express MHC class 1 chain-related protein A (MICA)
|
TRUE
|
|
|
|
What do virus-infected cells express that Natural Killer cells recognize that Non-infected, healthy cells do not express?
|
MIC Ligands for NKG2D
|
|
|
|
Define the Complement System
|
A group of blood and cell surface proteins that are important to inflammatory and immune responses
|
|
|
|
What are the major biological roles of the complement system?
|
Label Microbial Pathogens for Destruction
Disrupt Membranes of Microbial Pathogens leading to lysis Recruit Inflammatory Cells Remove Immune Complexes (i.e when Ab's bind antigen, they have to be removed somehow from circulation) |
|
|
|
Which protein involved in the complement system is most important in the compliment cascade?
|
C3
Found in the highest concentration of all the complement proteins in the blood |
|
|
|
What 3 pathways lead to the activation of the complement system?
|
Alternative Pathway = 1st to act
Lectin Pathway = 2nd Classical Pathway = 3rd Result of Complement Activation - Cleavage of C3 to C3a and C3b. C3b covalently bound to surface components of pathogen |
|
|
|
Once activated, what specific events occur during the Compliment System?
|
Cleavage of C3 to C3a and C3b. C3b covalently bound to surface components of pathogen
|
|
|
|
Compliment system activation leads what events?
|
Recruitment of inflammatory cells
Opsonization of pathogens, Facilitated uptake and killing of by phagocytes Perforation of pathogen cell membrane ULTIMATELY: Death of Pathogen |
|
|
|
Describe the events of compliment activation via the alternative pathway
|
C3 Protein floating in plasma
Small amt. C3 hydrolyzed (if this happens near pathogen, C3 is split into C3a and C3b C3a = Inflammatory Mediator C3b = Covalently Attaches to Glycoproteins C3b associates with other proteins = compliment cascade continues with C5b and C5a (pro-inflammatory) C6, C7, C8 and C9 form a complex called the MAC Comples (=Membrane Attack Complex) come in and form a hole in pathogen membrane |
|
|
|
Where does the compliment system activity occur?
|
Surface of pathogen (=your cells are not affected)
|
|
|
|
Whats the most important step in compliment activation?
|
C3b attaching to pathogen surface
|
|
|
|
The Membrane Attack Complex is made up of what proteins in the Compliment Activation Cascade?
|
C5b, C6, C7, C8, C9
|
|
|
|
When does the Membrane Attack Complex form in the Compliment Activation Cascade?
|
After activation via any of the 3 activation pathways (Alternative, Lectin, Classical)
|
|
|
|
What protein activates Membrane Attack Complex formation by C6, C7, C8, C9?
|
C5b
|
|
|
|
What is Mannose' role in Compliment Activation by Lectin Pathway?
|
Mannose-binding lectin binds glycoproteins/lipids w/ mannose (= not common to human cells but common in bacterial cells)
=Proteases activated that produce C3 convertase which makes more C3a and C3b |
|
|
|
List the Acute Phase Proteins
|
C-reactive Protein (CRP)
Mannose-binding Lectin (MBL) Serum Amyloid Proteins (SAP) Compliment System Proteins Fibrinogen |
|
|
|
What is Opsonization? What happens when we use two or more Opsonins?
|
Opsonization = When antigens are bound.
When we use two or more Opsonins, we get very enhanced binding and phagocytosis |
|
|
|
What are the ways the Compliment System is Regulated?
|
It has to be activated
Generation and Inactivation of C3b Compliment receptors on cells C1 Inhibitor Decay Accelerating Factor (DAF) CD59 (=on cells. Protects against compliment activation) |
|
|
|
Complement System activation is associated with what Auto-immune and Immune-mediated Diseases?
|
Inflammation
Vasculitis Glomerulonephritis (renal disease) Intra/Extravascular Hemolysis |
|
|
|
What are the consequences of deficient production of C1 inhibitor?
|
Deficient C1 Inhibitor (= Inhibits C1 Activation) = We get Increased Compliment Activation = More Susceptible to infections
|
|
|
|
A lack of which protein means we can't form the Membrane Attack Complex?
|
C3 (We can still form the MAC complex without C2)
|
|
|
|
What clinical disease is expected in a patient deficient in compliment proteins CD59 and DAF?
|
Paroxysmal Noctural Hemoglobinuria = dark urine in morning bc complement activated on eurythrocytes = lysis = show up in urine
|
|
|
|
What is the most positively charged human immunoglobulin in the body?
|
Albumin, but it has different forms, like gamma, beta, alpha2 and alpha 1 that allow it to be more negatively charged.
|
|
|
|
List the relative charge of each of the following immunoglobulins: IgG, IgA, IgM, IgD
|
From more Negative to Positive:
IgG, IgA, IgM, IgD |
|
|
|
What is an immunogen?
|
Any substance capable of inducing an immune response
|
|
|
|
What is an antigen?
|
Anu substance that can serve as the target of an immune response
|
|
|
|
What are the main determinants of immunogenicity? (=the ability of a substance to provoke an immune response)
|
Large Size
Chemical Complexity Solubility and Biodegradability Forein |
|
|
|
Define Hapten:
|
A substance, usually very small, that ALONE is not immunogenic (alone, it doesn't cause an immune response) but after conjugation to a carrier protein or cell becomes immunogenic. Ab formed can then bind the hapten, carrier, or both.
|
|
|
|
Define Epitope
|
The portion of the antigen that is bound by the Antibody or T cell. (the portion of the antigen that determines its immunogenicity)
Epitopes can be different on the same molecule or repeated on the same molecule |
|
|
|
Whats the difference between a Linear Epitope and a Discontinuous Epitope?
|
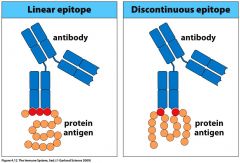
|
|
|
|
Describe the response of an antibody at initial contact and then when it contacts antigen again
|
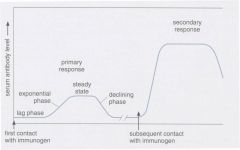
|
|
|
|
Memorize the following chart:
|
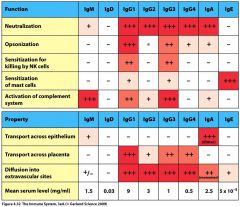
|
|
|
|
Where do the hyper-variable regions lie in the structure of an immunoglobulin?
|
The hyper-variable regions are in the part of the immunoglobulin available for contact with antigen
|
|
|
|
Define Isotype
|
Variants present in all members of the species
|
|
|
|
Define Allotype
|
Variants that differ between members of the species
|
|
|
|
Define Idiotype
|
Variants due to the hypervariable regions of Ig and TCR
|
|
|
|
What are some of the forces that act to bring Ab into contact with Antigen?
|
Hydrogen bonding
Electrostatic Van Der Waals Hydrophobic |
|
|
|
What are the events leading up to antibody secretion by a plasma cell?
|
Antigen recognition induces expression of effector molecules by T cell which activates B cell
B-cell proliferation Differentiation to resting memory cells and Ab-secreting plasma cells |
|
|
|
How are antibodies used in clinical medicine?
|
1. Auto-reactive Ab in autoimmune diease
2. Vaccination and use of pre-formed Ab 3. Monoclonal Ab 4. Laboratory Serology |
|
|
|
Describe Immunoflourescence
|
Produce reagents specific for what we're measuring in a serum sample, for ex, use an Anti IgA = an Ab specific for IgA
The dye is labeled and allows you to video it. |
|
|
|
Whats the idea behind vaccination?
|
Give pt. vaccine --> produces a primary immune response --> Lots of memory cells produced --> when person encounters pathogen in real life, the response to pathogen will be quicker bc memory cells already present (primary IgG Abs will be present now, which are a better isotype for the immune response because they are More specific than monoclonal Abs)
|
|
|
|
How can we mass produce monoclonal Abs for market?
|
Infect an animal with antigen
Remove spleen, which should have lots of B cells proliferating Spleen will produce monoclonal Abs, however these Abs are usually different that the human form of the same Ab, so thay have to be altered before they will work in humans |
|
|
|
Define Serology
|
Lab detection of specific serum Abs
For ex. ELISAs |
|
|
|
What are ELISAs?
|
Enzyme-linked immunosrobent assay
Used to detect an semi-quantitate antigen or Ab Reagent Ab labeled with detector so we can quantitate it. |
|
|
|
A pt. presents with the following laboratory values. What is your initial diagnosis?
IgA = 5 mg/dL (Normal = 40-60) IgG = 1320 (Normal = 800-1400) IgM = 72 (Normal = 30-120) |
Pt. has immune deficiency with selective IgA deficiency (this is the most common immune deficiency)
|
|
|
|
A pt. is having sinusitis, continuous cold, and nasal blockage after having moved to a new city. If his Immunoglobulin Levels are as follows, whats your initial diagnosis?
IgA = 120 (N=76-390) IgE = 1500 (N=0-380) IgG = 1280 (N=650-1500) IgM = 300 (N=40-345) |
Patient's IgE is high, meaning he may be experiencing an allergic response since IgE is involved in sensitization of mast cells
|
|
|
|
List the Immunoglobulin proteins in order from lowest serum concentration to highest.
|
"Ed Did Great Getting Mary Awake 2 GoGo"
(Just remember the G's go in order from lowest conc. = G4 to G3 to G2 to G1 = highest conc.) IgE -- IgD -- IgG4 -- IgG3 -- IgM -- IgA -- IgG2 -- IgG1 |
|
|
|
What is the role of the Fc Receptor?
|
Complement System Activation
Neutrophils etc. will have them. |
|
|
|
Whats the difference between secretory IgA and Serum IgA?
|
Secretory IgA = main Immunoglobulin found in mucous secretions like nasal, tears, saliva
Serum IgA = in serum |
|
|
|
Compliment System Events
|
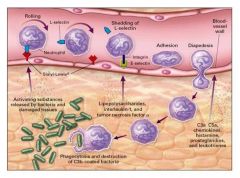
|
|
|
|
What is the signaling complex of the T Cell? (i.e. what part of the T Cell reads whether a cell is an antigen or not?)
|
CD3
|
|
|
|
Define T Cell
|
Primary initiator, coordinator, regulator of Adaptive Immune response.
Both cellular and humoral immunity depend on T cell Function |
|
|
|
What are the three major functions of T cells?
|
"Help, Kill, Regulate"
1. Helper Cell - help B cells make Abs. Secrete cytokines that recruit immune cells and activate immune and non-immune cells 2. Killer Cells - Mediate direct apoptosis of virus-infected cells and tumor cells 3. Regulatory Cell - prevent immunopathology by suppressing immune responses |
|
|
|
Where do T cells originate, and where do they migrate to?
|
Originate in Bone Marrow, migrate to Thymus where they undergo selection
Become active in Lymphoid organ after they encounter antigen Once activated, T Cells go to site of infection and perform their effector function |
|
|
|
Describe the T Cell Receptor
|
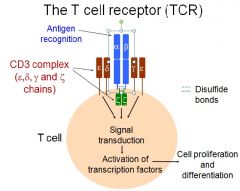
Antigen Recognition occurs at alpha and beta sites
CD3 complex on either side of alpha/beta read whether T cell is seeing an antigen Disulfide bonds attach the signaling proteins to the receptor |
|
|
|
T/F: The T Cell Receptor (TCR) only recognizes antigen presented by MHC (=Major Histocompatibility Complex)?
|
TRUE
T Cell Receptors can either be CD4+ or CD8+ CD4+ binds MHC class II CD8+ binds MCH class I To remember: CD4 x 2 = 8 CD8 x 1 = 8 |
|
|
|
In what places throughout the body can the T Cell Receptors recognize MHC Class I and MHC Class II molecules?
|
Thymus (As T cell progenitors)
Secondary Lymphoid Organs (as mature Naive T Cells) Periphery (skin, intestine, lungs, etc. as activated T cells) |
|
|
|
What percentage of T Cells Fail to become selected and activated in the thymus, and are thus apoptosed?
|
95%
|
|
|
|
T/F: Only self antigens are produced in the thymus
|
TRUE
|
|
|
|
T/F: The only antigen that T Cells test themselves with are other anitgens
|
TRUE
|
|
|
|
Where in the thymus does positive T Cell selection occur? Negative T Cell selection?
|
Positive selection occurs in the cortex of the thymus
Negative selection occurs in the Medulla of the thymus |
|
|
|
How does positive T cell selection in cortex of thymus work?
|
T cells that recognize antigen presented by MHC survive then go on to undergo negative selection.
If they don't recognize MHC, the T cell is deleted. |
|
|
|
Once a T cell in the thymus recognizes an antigen presented by MHC, it then goes on to negative selection - describe how negative selection works
|
T cells that recognize "self" peptides are deleted, because we don't want T cells attacking our own cells! This is a safety mechanism
If the T cell does not recognize the "self" peptide, it survives and leaves the thymus as a mature naive T cell. |
|
|
|
What is the determining factor in T Cell Negative selection in the medulla of the thymus
|
Binding affinity (Remember; negative selection is when T Cell is tested to see if it binds "self")
T Cell binds Moderately or Loose = Lives T Cell binds tightly = It is Killed |
|
|
|
Define the concept of central tolerance
|
Positive and Negative selection generate Central Tolerance (= occurs in thymus) vs. Peripheral Tolerance that plays a role in preventing autoimmunity
|
|
|
|
Regulatory T Cells (Tregs) do what?
|
Suppress Adaptive Immune Response by suppressing action of CD4 and CD8 cells
|
|
|
|
Negative selection generates a small number of what type of cell that aids in inactivating self-reactive T cells?
|
CD4+ Regulatory T cells (Tregs)
Negative Selection = T cells presented with "self" antigen. Those that bind "self" are killed, however a small number survive and become CD4+ regulatory T cells (Tregs) that REGULATE (!) those cells that may have escaped these regulatory mechanisms to prevent T cells from attacking self. |
|
|
|
Tregs are generated in what two ways?
|
1. Natural Tregs generated via Negative Selection (mediate peripheral tolerance)
2. Induced Tregs are generated in the periphery (ALSO mediate peripheral tolerance) |
|
|
|
T/F: Both Natural and Induced Tregs mediate peripheral tolerance
|
TRUE
|
|
|
|
Lack of Tregs results in what condition? What are the signs/symptoms?
|
A defect in peripheral tolerance and an inability to inactivate T and B cells that escape central tolerance
Signs/Symptoms: watery diarrhea, failure to thrive, dermatitis, autoimmune diabetes in infnacy Treatment = bone marrow transplant |
|
|
|
T/F: T Cells circulate throughout the blood and through secondary lymphoid tissue in search of Antigen presenting cells presenting something it can recognize.
|
TRUE
|
|
|
|
Where do T cells mature?
|
Blood
|
|
|
|
What types of Antigen-Presenting Cells occur in the Lymph Nodes?
|
1. Professional Antigen presenting cells (macrophages, dendritic cells)
2. B Cells |
|
|
|
What types of Antigen-Presenting Cells occur in the Periphery (as opposed to the lymph node)?
|
1. Professional Antigen-presenting cells (same as in lymph node - macrophages, dendritic cells
2. Non-Immune cells, such as virus-infected epithelial cells |
|
|
|
What are the four CD4+ T Helper Cell Subsets?
|
Th1
Th2 Treg Th17 |
|
|
|
What is the function of Th1 cells, a subset of CD4+ T Helper cells?
|
Initiate/Regulate CELLULAR Immunity (vs. Th2 cells which do HUMORAL immunity)
IFN-gamma (AKA Type II Interferon) = a cytokine that helps to activate macrophages and help B cells make Ab (IgG1). In this way, Th1 cells also help with humoral immune response |
|
|
|
What is the function of Th2 cells, a subset of CD4+ T Helper cells?
|
Initiate/Regulate HUMORAL immunity (vs. cellular immunity regulated by Th1 cells)
Recognize peptide presented by B Cells in lymph nodes Secrete cytokines that help B Cells |
|
|
|
What are two anti-inflammatory cytokines that inhibit Th1 development?
|
IL-10 and transforming growth factor beta (TGF-beta)
|
|
|
|
What do Th17 Cells do?
|
Produce pro-inflammatory cytokines (= play role in inflammatory bowel disease, Rheumatoid Arthritis, MS and Asthma)
IL-17, IL-21, IL-22 Activate macrophages, fibroblasts, endothelia and epithelia to promote inflammation |
|
|
|
When you see Th17 Cell, what process is most likely occurring?
|
Inflammation
|
|
|
|
What are the two types of CD4+ T Cell Receptors?
|
Alpha-beta and gamma-delta = the regions that bind (vs the CD3 region of the protein)
vs. CD8+ T cells |
|
|
|
CD8+ cells recognize antigen presented in the context of MHC I or MHC II?
|
MHC I
|
|
|
|
Note the different roles of CD4 and CD8 Cells
|
|
|
|
|
List the different CD4+ Effector T cells, the Cytokines produced by each CD4+ T Cell effector, and their cellular target.
|

CD4+ Effector T Cells Include: Th1, Th2, Th17, Treg
NOTE: There is an error on picture; Treg effector T cells produce IL-10 and TGF-beta, NOT IL-1. |
|
|
|
CD4+ T Cell Effector Th1:
Cytokines Produced? Cellular Target? |
Cytokines Produced =
IL-2 IFN-gamma |
Cellular Target =
Macrophages CD8 T Cells |
|
|
CD4+ T Cell Effector Th2:
Cytokines Produced? Cellular Target? |
Cytokines Produced =
IL-4, 5, 6, 10, 13 |
Cellular Target =
B Cells Mast Cells Eosinophils |
|
|
CD4+ T Cell Effector Th17:
Cytokines Produced? Cellular Target? |
Cytokines Produced =
IL-17, 21, 22 |
Cellular Target =
Fibroblasts Endothelial Cells Epi cells |
|
|
CD4+ T Cell Effector Treg:
Cytokines Produced? Cellular Target? |
Cytokines Produced =
IL-10, TGF-beta |
Cellular Target =
Th1, Th2, Th17 |
|
|
T Cell Cytokine IL-2:
Major Source? Major Effect? |
Major Source =
CD4+ cells |
Major Effects =
Proliferation of T, B, NK cells |
|
|
T Cell Cytokine IL-4:
Major Source? Major Effect? |
Major Source =
Th2 T Cells |
Major Effects =
B-cell Proliferation IgE Production Induces Th2 Cell development |
|
|
T Cell Cytokine IL-10:
Major Source? Major Effect? |
Major Source =
Th2 T Cells Treg |
Major Effects =
Inhibits macrophages and dendritic cells Inhibits development of Th1 cells |
|
|
T Cell Cytokine IL-12:
Major Source? Major Effect? |
Major Source =
Th1 T Cells |
Major Effects =
Inhibits development of Th2 cells Promotes development of Th1 cells |
|
|
T Cell Cytokine IL-13:
Major Source? Major Effect? |
Major Source =
Th2 Cells Basophils Mast Cells |
Major Effects:
IgE Production Increased Mucous Production |
|
|
T Cell Cytokine IL-17:
Major Source? Major Effect? |
Major Source =
Th17 |
Major Effects =
Induce inflammatory cytokines and recruit neutrophils |
|
|
T Cell Cytokine INF-gamma:
Major Source? Major Effect? |
Major Source =
Th1 Cells |
Major Effects =
Macrophage Activation Promotes Th1 Development Promotes MHC Expression |
|
|
T Cell Cytokine TGF-beta:
Major Source? Major Effect? |
Major Source =
Treg |
Major Effects =
Inhibits Lymphocyte Activation Anti-inflammatory Promotes IgA Production |
|
|
MHC Class I is composed of:
A. alpha chain non-covalently associated with B2-microglobulin B. alpha chain covalently associated with B2 microglobulin C. Covalently associated alpha and beta chains D. non-covalently associated beta chains |
A. MHC Class I is composed of:
Alpha chain non-covalently associated with B2-microglobulin |
|
|
|
What are Exogenous antigens loaded onto?
|
MHC Class II molecules in an endosome
|
|
|
|
Why is tissue transplant difficult from patient to patient?
|
MHC molecules are very different = why tissue transplant is difficult
|
|
|
|
What is the major histocompatibility complex?
|
The genetic region that contains the MHC I and MHC II genes
|
|
|
|
What determines compatibility between individuals during tissue and organ transplantation?
|
MHC I and MHC II determine Histocompatibility
|
|
|
|
What do MHC molecules do?
|
Present peptides to T cells
|
|
|
|
Describe the relative size of epitope MHC Class I and MHC class II molecules can bind
|
MHC I binds peptides (epitopes) 7-15 AAs in length, while MHC II binds peptides (epitopes) 10-30 AAs in length
|
|
|
|
Picture the MHC class I and MHC class II molecules
|
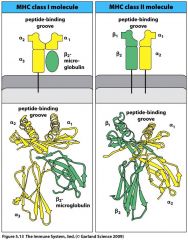
|
|
|
|
Antigens present outside the cell are presented to CD4+ T cells by what? (Exogenous antigens taken into cell, degraded, presented)
|
MHC II
|
|
|
|
Antigens present inside the cell are presented to CD8+ T cells by what? (Endogenous antigens in cytosol degraded, presented)
|
MCH I
|
|
|
|
Antigens outside cell are presented on which MHC?
|
MHC II
|
|
|
|
Antigens inside cell are presented on which MHC?
|
MHC I
|
|
|
|
How do antigen-presenting cells take in antigen?
|
Receptor-mediated endocytosis:
-Antigen coated with complement proteins --> Antigen taken in by complement receptor -Fc Receptor Antigens can ALSO get in by fluid-phase endocytosis |
|
|
|
List the pathways dendritic cells use to get antigens into them
|
1. Receptor-mediated endocytosis (MHC II = CD4+)
2. Macropinocytosis (MHC II = CD4+) 3. Viral Infection (MHC I = CD8+)) 4. Cross-presentation after phagocytic or macropinocytic uptake (MHC I) 5. Transfer from incoming dendritic cell to resident dendritic cell (MHC I) |
|
|
|
Where are peptides that bind MHC Class II Molecules generated?
|
Acidified Endocytic Vesicles
|
|
|
|
Acidification of vesicles does what to proteases which degrade antigen into peptide fragments?
|
Acidification of vesicles ACTIVATES proteases to degrade antigen into peptide fragments
Vesicles containing peptides then fuse with vesicles containing MHC Class II molecules |
|
|
|
Peptides that bind MHC II molecules are generated in what?
|
Acidified Endocytic Vesicles
|
|
|
|
T/F: Acidification of vesicles activates proteases to degrade antigen into peptide fragments
|
TRUE
|
|
|
|
Where does processing of antigens for presentation on MHC II occur?
|
Phagolysosome
|
|
|
|
Picture the process of antigen presentation on MHC II
|
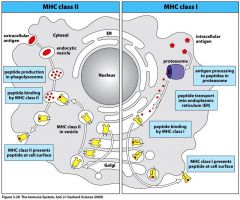
|
|
|
|
T/F: Viruses use the cell's innate machinery to replicate, thus viral proteins are synthesized in the ER and golgi, and assembled in the cytosol
|
TRUE
Viruses are obligate intracellular parasites |
|
|
|
Is a protein transporter required to get protein peptides into the ER?
|
Yes,
Proteins required for peptide transport into the ER for loading into MHC I molecules |
|
|
|
Where does processing of antigens for presentation on MHC I occur?
|
ER
(Vs. for MHC class II, which occurs in phagolysosome) |
|
|
|
T/F: An MHC molecule can present a variety of peptides
|
TRUE
Different from Ab's, which can only bind one thing = Ab's more specific than MHC's |
|
|
|
T/F: A T Cell receptor can bind a variety of peptides presented by an MHC molecule
|
FALSE
B and T Cell receptors stick to one peptide |
|
|
|
What types of cells express MHC Type I?
|
T cells
B Cells Macrophages Dendritic Cells Neutrophils ALSO: Thymic Epithelium Liver Hepatocytes Kidney Epi Brain |
|
|
|
What Cells Express MHC Class II?
|
T Cells, when activated
B Cells Macrophages Dendritic Cells Thymic Epithelium NOT NEUTROPHILS, Liver hepatocytes, Kidney Epithelium, Brain |
|
|
|
What is Bare Lymphocyte Syndrome?
|
A disease in which EITHER MCH class I or II molecules NOT expressed on cell = Immunodeficiency
Deficiency/Lack of MHC Class I = Lack of CD8+ T cells = Susceptible to intracellular pathogens Lack of MHC Class II = Lack of CD4+ = susceptible to extracellular pathogens |
|
|
|
Define Human Leukocyte Antigen (HLA)
|
Genetic designation for human MHC
For ex. HLA-A*0201 HLA = Loci, 0201 = Alleles = we want to match as many of these between donor/recipient as possible |
|
|
|
What are major histocompatibility complex molecules?
|
Highly Polymorphic Proteins that bind antigens and present them to T cells (HMC I and MHC II)
|
|
|
|
Define Polymorphism
|
Existence of different variants of a gene; 2 or more different forms or alleles of a gene
|
|
|
|
Define Allele
|
Natural variants of a gene; one of several alternate forms of a gene at a single locus (HLA-A has 506 alleles etc.)
|
|
|
|
Define Haplotype
|
The set of polymorphic genes carried on a single chromosome. Humans inherit two haplotypes, one from each parents.
|
|
|
|
T/F: MHC isotypes differ in the extent of their polymorphism
|
TRUE
Polymorphism can mean you are expressing two forms of one gene |
|
|
|
Which MHC isotypes encode MHC Class I?
|
HLA-A
HLA-B HLA-C (Not as important to know: HLA-E, HLA-F, HLA-G) |
|
|
|
Which MHC isotypes encode MHC Class II?
|
HLA-DP
HLA-DQ HLA-DR (Not as important to know: HLA-DM, HLA-DO) |
|
|
|
What characteristic of HLA genes has important implications for tissue transplation?
|
The fact that some HLA genes are highly polymorphic = there are different variants of the gene
|
|
|
|
HLA-A Encodes what?
|
MHC class I alpha chain
(Beta chain doesn't really contribute much - just there for structural support) |
|
|
|
HLA-DR Encodes what?
|
HCM Class II alpha and beta chains
(unlike the important HLA-A, HLA-B, and HLA-C mentioned in class, which only encode alpha chain) |
|
|
|
T/F: Expression of MHC alleles is co-dominant
|
TRUE
Results in heterozygosity - inherit dif. alleles from both parents = both alleles expressed |
|
|
|
Why is the fact that Expression of MHC alleles is co-dominant = heterozygosity good for a person?
|
Good b/c this means we have immunity to many dif. pathogens.
If we were homozygous, we wouldn't be as immune to as many things. |
|
|
|
T/F: All MHC molecules can be expressed on the cell surface at any given time
|
TRUE
All of them can bind and present a range of peptides |
|
|
|
Besides polymorphism, what else contributes to the diversity of MHC molecules expressed by an individual?
|
Polygeny
|
|
|
|
Which regions of the MHC I molecule are the most diverse in terms of amino acid variability?
|
alpha 1 and alpha 2 domain = most diverse
|
|
|
|
Which regions of the MHC II molecule are the most diverse in terms of amino acid variability?
|
alpha 1 and beta I regions = most diverse
|
|
|
|
Where is variation between MHC allotypes concentrated in MHC I and MHC II molecules?
|
Variation occurs at the sites that bind peptide and the TCR
|
|
|
|
Whats one reason some people survive diseases that others don't?
|
Diversity of MHCs in the person
|
|
|
|
T/F: MHC heterozygosity has no affect in the progression of AIDS in HIV-1 infected people
|
FALSE.
MHC Heterozygosity delays the progression of AIDS in HIV-1 infected people Inc. Heterozygosity = Longer Survival |
|
|
|
What cell confers antigen specificity?
|
Lymphocytes
|
|
|
|
Where do WBCs undergo hematopoesis?
|
Bone Marrow
|
|
|
|
Whats the difference between B cells and T cells in their maturation processes?
|
T cells leave Thymus to mature and become functional T cells
B cell precursors remain in Bone Marrow to mature (from pluripotent stem cells) |
|
|
|
Describe how Lymphocytes confer antigen specificity
|
1. Cells give rise to lots of lymphocytes that circulate - each has a dif. cell surface receptor
2. Receptors of only a few lymphocytes interact with any given pathogen 3. Pathogen-reactive lymphocytes triggered to divide/proliferate 4. Pathogen-activated lymphocytes differentiate into effector cells that eliminate pathogen |
|
|
|
Describe the structure of Immunoglobulin
|
2 Identical light chains
2 Identical heavy chains These two things make up the antigen binding site = where the variability occurs |
|
|
|
Variable regions of immunoglobulin are encoded by what?
|
gene segments:
2 gene segments code for te 2 light chains 3 gene segments code for the 2 heavy chains SO cells, as they develop, have many of these gene segments to choose from |
|
|
|
How does a B Cell produce a Kappa Light Chain?
|
The variable part of the LIGHT chain is encoded by two dif. gene segments: V Kappa and J Kappa.
V Kappa = 40 to choose from J Kappa = 5 to choose from B Cell chooses one of each to form the coding message |
|
|
|
What genes help out in the formation of B Cell Kappa Light Chain Rearrangement?
|
RAG-1 and RAG-2
Recombination Activating Genes 1 and 2 |
|
|
|
What do RAG-1 and RAG-2 do?
|
Encode proteins/ enzymes involved in function of choosing gene segments, splicing them together, taking out everything else not needed.
|
|
|
|
The B cell is teh only cell to do what in terms of immunoglobulins?
|
B Cell is the only cell that can Re-arrange gene segments, which is why the B cell is the only one to make Antibody
|
|
|
|
What determines the isotype of the Immunoglobulin?
|
Constant region of the heavy chain
|
|
|
|
What are one of the 1st antibodies produced by B cells?
|
IgM
|
|
|
|
The heavy chain is encoded by how many VARIABLE segments?
|
3
V, D, J (where as Light chain has 2 variable segments, V and J) |
|
|
|
How is Antibody diversity created?
|
Developing B cell receptors form from gene segments
Variable regions encoded by V, D, J segments Random combination of segments produces greater variability |
|
|
|
What are additional ways Antibody diversity can be created?
|
Differences in the ways genes come together
Terminal Deoxynucleotidyl transferase (TdT) Light and Heavy chain combinations Somatic Hypermutations |
|
|
|
How does Terminal Deoxynucleotidyl Transferase (TdT) produce increased Antibody diversity?
|
TdT = An Enzyme active in developing Lymphocytes
Adds random nucleotides to segments |
|
|
|
Whats the difference between B cells and T cells diversity in terms of affinity for antigens?
|
B cells can undergo somatic hypermutations = point mutations increase their diversity for antigens they recognize = dif. antigen recognized
After being cloned, T cells still have the SAME specificity for antigen |
|
|
|
How do Somatic Hypermutations produce increased Antibody diversity?
|
Point mutations occur in receptor molecules = changes affinity for receptor
|
|
|
|
What are the surface Immunoglobulin receptors?
|
IgM or IgD
|
|
|
|
Which Immunoglobulin receptors do intracellular signaling?
|
IgA (Ig-alpha) and IgB (Ig-beta)
-Can be phosphrylated and then do intracellular signaling Accessory proteins = mechanism of action for signal being carried from membrane to nucleus = to divide, produce cytokines etc. |
|
|
|
What Co-stimulatory molecules are involved in B-cell Activation?
|
CD21 and CD19
Part of the co-receptor complex |
|
|
|
What are the 5 major Antibody Isotypes?
|
IgM
IgD IgC IgE IgA |
|
|
|
If a cell is making IgM, hwo do we get production of the other isotypes?
|
When B cell activated, many of the clones produced will undergo isotype switching = switch from making IgM to making some other isotype
|
|
|
|
Isotype switching does what to the VDJ segments?
|
Brings them closer
|
|
|
|
Both somatic hypermutation AND isotype switching are dependent on what?
|
AID, Activation-Induced Cytidine Deaminase
|
|
|
|
AID, Activation-Induced Cytidine Deaminase is expressed only in what?
|
ACTIVATED B Cells
|
|
|
|
How does AID, Activation-Induced Cytidine Deaminase work?
|
Makes Knick in DNA = DNA Degraded = VDJ Segments for Variable Region coem close = Everything between them is gone = Now daughter cell will make a dif. Ig
|
|
|
|
What influence do T Cells have on isotype switching? (i.e. B cells making a dif. Ig?
|
T Cells produce cytokines that influence isotype switching (for ex. interleukin 4 = induces switching to IgE = Why TH2 cells imp. in allergic reactions)
|
|
|
|
What determines what isotype is produced when a B Cell undergoes isotype switching?
|
Helper T Cells + B Cells = Activation of B cell so it can do isotype switching
CD40 on B Cell AND CD 40 on T Cell |
|
|
|
Whats the major function of IgM?
|
Activation of Complement System
|
|
|
|
Whats the major function of IgD?
|
not on chart
|
|
|
|
Whats the major function of IgG1?
|
Neutralization
Opsonization Sensitization for killing by NK cells Activation of Complement System NOTE: Transported across placenta Diffusion into Extravascular Sites |
|
|
|
Whats the major function of IgG2?
|
Neutralization
Note: Diffusion into Extravascular Sites |
|
|
|
Whats the major function of IgG3?
|
Neutralization
Opsonization Sensitization for Killing by NK cells Activation of Complement System Somewhat: Sensitization of Mast Cells Note: Transported across Placenta Diffusion into Extravascular Space |
|
|
|
Whats the major function of IgG4?
|
Neutralization
Note: Transported across Placenta Diffusion into Extravascular Space |
|
|
|
Whats the major function of IgA?
|
Neutralization
Somewhat: Activation of complement system Note: Transport Across Epithelium Diffusion into Extravascular Sites |
|
|
|
Whats the major function of IgE?
|
Sensitization of Mast Cells
Note: Diffusion into Extravascular Sites |
|
|
|
After antigen exposure, what is the primary response made up of mostly, in terms of Immunoglobulins isotype?
|
IgM isotype
|
|
|
|
After antigen exposure, what is the secondary response made up of mostly, in terms of Immunoglobulins?
|
IgG isotype
|
|
|
|
What is Burkett's Lymphoma?
|
B Cell Malignancy caused by a virus = produces mono
Infects B cells, and, via chromosomal rearrangement, induces cancer |
|
|
|
During B Cell replication, translocation from chromo 8 to chromo 14 results in what?
|
Chromo 14 contains genes for the heavy chain of Ab. If transolocation puts a protooncogene on chromo 14, it can be cancerous
|
|
|
|
Protection in the immune system of a developing fetus or neonate comes from what?
|
IgG from mother
5-6 months later, babies' IgG goes down, but by this time baby can develop own Abs. |
|
|
|
When are babies most susceptible to infections?
|
After birth, before they've begun to fully make their own IgG (i.e. couple months after birth)
|
|
|
|
What happens if someone doesn't have RAG-1 or RAG-2?
|
Severe Immunodeficiency = don't produce enough T cells or B cells
= "Bubble Boy Syndrome" |
|
|
|
What happens if someone doesn't have any AID, Activation-Induced Cytidine Deaminase?
|
No isotype switching = Lots of IgM, not a lot of others = very susceptible to infections
|
|
|
|
When dendritic cells recognize pathogen via pattern recognition, what are some of the things they do?
|
Undergo functional maturation
Begin to present peptide on their cell surface Up-regulate co-stimulatory molecules |
|
|
|
Chemokines secreted by what Cell types recruit dendritic, T cells and B cells to lymph nodes?
|
Lymph node stromal cells
Dendritic Cells |
|
|
|
What is the most potent antigen presenting cell?
|
Dendritic Cells
|
|
|
|
What are some examples of dendritic cells on the skin? Tissues? Blood?
|
Skin: Langerhan's and Dermal dendritic cells
Tissues: Myeloid dendritic cells Blood: Plasmacytoid dendritic cells |
|
|
|
What do Langerhan's Cells (epidermal dendritic cells) preferentially induce; cellular immunity or humoral immunity?
|
Cellular Immunity
|
|
|
|
What do Dermal dendritic cells (epidermal dendritic cells) preferentially induce; cellular immunity or humoral immunity?
|
Humoral Immunity
Secrete cytokines that promote the development of Ab responses |
|
|
|
Whats the major Type I Interferon producing cell in the blood?
|
Plasmacytoid Dendritic Cell
Very good at detecting viruses in blood Make Type I Interferons (IFN-alpha, INF-beta) that play a major role in anti-viral defense |
|
|
|
T/F: Myeloid dendritic cells are found only in lung and intestinal cells
|
FALSE
Myeloid dendritic cells are found in almost all tissues If from monocytes, they are inflammatory monocytes |
|
|
|
Dendritic Cells collect antigens and migrate where to present a now processed antigen?
|
Lymph Node
|
|
|
|
Immature Dendritic cells in the peripheral tissues seek out antigen, then what?
|
They mature as they migrate to lymph node where they present a processed antigen
|
|
|
|
Dendritic cells change their morphology and function after collecting antigens: What is the morphology of an immature dendritic cell?
|
MHC II co-localizes with lysosomal marker
|
|
|
|
Dendritic cells change their morphology and function after collecting antigens: What is the morphology of a dendritic cell after antigen uptake?
|
MHC class II is loaded with antigen and moves OUT of lysosome to cell surface = this is a major change in the membrane
|
|
|
|
What things occur during dendritic cell maturation?
|
1. Endocytosis is down-regulated
2. Antigens undergo processing into peptides 3. Peptides loaded onto MHC molecules 4. MHC molecules move to cell surface and display peptide 5. Dendritic cells down-regulate chemokine receptors that promote migration to inflammatory chemokines 6. Dendritic cells up-regulate chemokine receptors that promote migration to lymph node-derived chemokines 7. Dendritic cells up-regulate expression of co-stimulatory molecules (CD80 and CD86) |
|
|
|
What is Chemotaxis?
|
Process of directed migration down chemical gradient
ie infection = inflammation and release of inflammatory chemokines |
|
|
|
What chemokine receptors respond to the CCL5 chemokine?
|
Chemokine Receptors CCR1 and CCR5
|
|
|
|
During infection, dendritic cells leave tissues and migrate in response to what?
|
Lymphoid chemokines present in lymphatics and lymph nodes
|
|
|
|
What chemokine receptor respond to the CXCL12 (SDF-1, stromal-derived factor 1) chemokine?
|
Chemokine Receptor 4 (CXCR4)
|
|
|
|
What chemokine receptor responds to the CCL19 and CCL21 chemokines?
|
Chemokine Receptor 7 (CCR7)
|
|
|
|
What do inflammatory chemokines do?
|
Recruit phagocytes to sites of infection where they destroy pathogens and/or acquire antigens for processing and presentation to T Cells
|
|
|
|
During infection, What do professional antigen-presenting cells respond to?
|
Lymphoid Chemokines = directs them to lymph nodes for interaction with T cells
|
|
|
|
T/F: T cells can access lymph from blood and lymph
|
TRUE
|
|
|
|
Once antigen-presenting cells are in the lymph node, whats the next step?
|
T Cells enter lymph node, crossing High Endothelial Vacuoles, and monitor the antigens presented by macrophages and dendritic cells
If T Cells don't encounter specific antigen, they leave T Cells that encounter specific antigen proliferate and differentiate into effector cells |
|
|
|
What processes are needed to activate T cells once they encounter a specific antigen?
|
Adhesion (to antigen-presenting cell)
TCR-MHC Peptide Co-Stimulation Cytokine IL-2 |
|
|
|
Integrins and Adhesion molecules are composed of what?
|
alpha and beta chains
|
|
|
|
What is LFA-1?
|
Leukocyte Function Associated Antigen 1
Integrin expressed by T cells that allows binding to the adhesion molecule ICAM-1 on Antigen Presenting Cells |
|
|
|
Picture Adhesion, with LFA-1 and ICAM-1
|
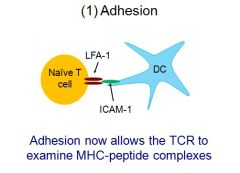
|
|
|
|
Without expression of CD18, what happens?
|
T cells can't be activated (LFA-1)
Macrophages can't get out of blood to access sites of infection (MAC-1 binds ICAM1 expressed on endothelial cells) i.e. without CD18, MAC1 can't interact with ICAM1 |
|
|
|
What is Leukocyte Adhesion Deficiency (LAD)?
|
Defect in the beta subunit of MAC1
|
|
|
|
During the TCR-MHC (T Cell Receptor/MHC) Peptide process of T Cell Activation, Antigen is present via which MHC to CD4+ cells?
|
MHC II
|
|
|
|
During the TCR-MHC (T Cell Receptor/MHC) Peptide process of T Cell Activation, Antigen is present via which MHC to CD8+ cells?
|
MHC I
|
|
|
|
T/F: Antigen presented via MHC's to CD4 o4 CD8 cells is sufficient for T cell Activation
|
FALSE
We must co-stimulate |
|
|
|
What does co-stimulation involve?
|
B7 Molecules = CD80 and CD86 Human Antigens
|
|
|
|
What do B7 molecules (CD80 and CD86) bind to?
|
CD28 on the T Cell
|
|
|
|
Whats the first cytokine produced after T cell activation and a major consequence of T Cell Receptor signaling?
|
IL-2
|
|
|
|
Activated T cells express IL-2 which does what?
|
IL-2 enhances T cell proliferation and differentiation in an autocrine fashion
|
|
|
|
What is MHC Restriction?
|
When the T Cell Receptor recognizes a complex of peptides bound to MHC = the T Cell Receptor is specific for the combination of peptide and MHC = BOTH have to fit
|
|
|
|
T/F: Antigen presentation to T Cells is MHC restricted
|
TRUE
alpha and beta chains can recognize antigen AND the class II molecule BUT, Not all antigens are presented this way (for ex. Super antigens) |
|
|
|
What do Super Antigens do?
|
Bridge the T Cell Receptor to MHC Receptor = Super antigen can bind = Polyclonal Activation
|
|
|
|
T/F: MHC Molecules are never on the surface unless antigen is presented
|
TRUE
|
|
|
|
What do B7 Molecules (CD80 and CD86) bind?
|
CD28 on BOTH CD4+ and CD8+ T Cells
|
|
|
|
What is Tolerance?
|
A way the immune system prevents autoimmunity
|
|
|
|
T/F: It is equally important to turn immune responses on and off
|
TRUE
|
|
|
|
B7 Co-stimulatory molecules CD80 and CD86 bind to CD28 on the T Cell to do what?
|
Activate T Cell
|
|
|
|
T/F: B7 Co-stimulatory molecules can also Suppress the T Cell?
|
TRUE
|
|
|
|
Shortly after being activated, what is Up-regulated on the T Cell?
|
CTLA-4 (Cytotoxic T Lymphocyte Antigen 4)
|
|
|
|
T/F: Antigen presentation to T Cells is MHC restricted
|
TRUE
alpha and beta chains can recognize antigen AND the class II molecule BUT, Not all antigens are presented this way (for ex. Super antigens) |
|
|
|
What do Super Antigens do?
|
Bridge the T Cell Receptor to MHC Receptor = Super antigen can bind = Polyclonal Activation
|
|
|
|
T/F: MHC Molecules are never on the surface unless antigen is presented
|
TRUE
|
|
|
|
What do B7 Molecules (CD80 and CD86) bind?
|
CD28 on BOTH CD4+ and CD8+ T Cells
|
|
|
|
What is Tolerance?
|
A way the immune system prevents autoimmunity
|
|
|
|
What are the two types of Tolerance?
|
Central and Regulatory
|
|
|
|
T/F: It is equally important to turn immune responses on and off
|
TRUE
|
|
|
|
B7 Co-stimulatory molecules CD80 and CD86 bind to CD28 on the T Cell to do what?
|
Activate T Cell
|
|
|
|
T/F: B7 Co-stimulatory molecules can also Suppress the T Cell?
|
TRUE
|
|
|
|
Shortly after being activated, what is Up-regulated on the T Cell?
|
CTLA-4 (Cytotoxic T Lymphocyte Antigen 4)
|
|
|
|
What does CTLA-4 (cytotoxic T Lymphocyte antigen 4 do?
|
After T cell is activated its up-regulated and LIMITS the T Cell's Proliferation
How: It has greater affinity for CD80 and CD86 on Antigen presenting cell than CD28 (which is on T cell) OVERALL EFFECT: T Cell Proliferation limited |
|
|
|
What do Tregs (Regulatory T Cells) do?
|
Mediate peripheral tolerance
|
|
|
|
What are the two types of Tregs (Regulatory T Cells)?
|
Natural Tregs (ie Naive T Cells) = Generated in thymus during positive and negative selection
Induced Tregs (ie Effector T Cells) = Generated in Periphery |
|
|
|
How do Tregs (Regulatory T Cells) work?
|
Secrete TGF-beta
Interact with T Cell Down-regulate/inhibit function of dendritic cell |
|
|
|
When antigen is presented by Non-Professional Antigen presenting cells, what happens?
|
T Cell Tolerance
|
|
|
|
Suppression of T Cell responses occurs by which mechanisms?
|
Antigen presentation in the absence of co-stimulation (= we need B7 = CD80/CD86) to activate T Cell
Tregs |
|
|
|
Differentiate between NaiveT Cell Response and Effector T Cell Response (=Th1 or Th2)
|
Naive T Cell's require co-stimulation (=via B7 = CD80/CD86)
Effector T Cells do NOT require co-stimulatory molecules to perform their functions |
|
|
|
What does B Cell (=Lymphocyte) activation require?
|
CD4 T cell help for T-dependent antigens
|
|
|
|
Whats the process of B Cell activation?
|
B cell endocytoses antigen bound to membrane immunoglobulin --> processes then presents antigen to CD4 T Cell
|
|
|
|
T/F: B Cells are professional antigen-presenting Cells
|
TRUE
Antigen processed then presented on MHC II |
|
|
|
What on the T Helper cell membrane and the B cell membrane is required for B cell activation and T Helper cell activation?
|
CD40 Ligand (T Helper cell) and CD40 (B Cell)
= Co-stimulation = now T cell knows its interacting with an antigen-presenting cell |
|
|
|
After B Cells internalize (via receptor mediated endocytosis) and process antigen bound to B cell receptor, what happens?
|
Antigen is processed and presented on MHC class II
|
|
|
|
How do T cells activate B cells?
|
T cells secrete cytokines, like CD40L (=CD40 Ligand) which attaches to CD40 receptor on B cell
|
|
|
|
T/F: Most antigens are T-dependent (-Thymus dependent) and T cell help is required for B cells to produce antibodies
|
TRUE
HOWEVER--> The exception is that B cells DO NOT need T Cell help to respond to Thymus-INDEPENDENT antigens |
|
|
|
What are the two antigens that can activate B cells without the help of T cells?
|
TI-1 and TI-2
HOWEVER--> Some cytokines can induce isotype switching in B cells in the absence of T cell help |
|
|
|
T/F: When B cells are activated by T-independent antigens without T Cell Help by TI-1 antigens, cytokines are not produced and thus IgM antibody is the only one produced (ie no isotype switching)
|
TRUE
|
|
|
|
T/F: When B cells are activated by T-independent antigens without T Cell Help by TI-1 or TI-2 antigens, there is no affinity maturation (=low affinity Abs)
|
TRUE
|
|
|
|
When B cells are activated by T-independent antigens without T Cell Help by TI-1 or TI-2 antigens, No memory response is generated.
|
TRUE
|
|
|
|
What is LPS?
|
A TI-1 antigen (=Thymus Independent Type 1 Antigen)
Thus, with exposure to LPS, we will get Anti-LPS IgM antibody response b/c LPS is a TI-1 antogen = they induce IgM Abs only |
|
|
|
What does LPS antigen (=a TI-1, Thymus Independent Type 1 antigen) interact with?
|
TLR-4 = B cell is interacted without any T Cell help = the definition of TI-1 and TI-2 antigens
|
|
|
|
T/F: LPS TI-1 antigen can induce an antibody response to other antigens
|
TRUE
For. ex. LPS can induce an antibody response to a flagella if it comes into contact with B cell |
|
|
|
What are TI-2 (=Thymus-Independent Type 2 antigens) antigens composed of?
|
Repetitive Carbohydrate (polysaccharide) or protein epitopes
|
|
|
|
What do TI-2 antigens result in?
|
Extensive BCR and co-receptor cross-linking
|
|
|
|
What is the Hib vaccine (H. Influenza) composed of?
|
Tetanus toxoid (=a T-dependent antigen) and H. Influenzae Polysaccharide (=a Gram neg. - capsule = a T-Independent Antigen)
|
|
|
|
Why is the HiB Vaccine effective? (=H. Influenza, or Hemophilus Influenza)
|
Tetanus toxoid converts the polysaccharide into a T-dependent antigen
|
|
|
|
How do cytokines work?
|
Autocrine
Paracrine Endocrine |
|
|
|
What are some examples of cytokines?
|
Hematopoietic Growth Factors
Chemokines Interleukins |
|
|
|
T/F: Cytokines are soluble but not membrane-associated
|
FALSE
|
|
|
|
T/F: Cytokines can be produced by non-immune cells such a epithelial and vascular cells in response to PAMPs
|
TRUE
|
|
|
|
What are the major groups of cytokines?
|
1. Pro-inflammatory
2. Anti-inflammatory 3. Interferons 4. Interleukins 5. Chemokines 6. Growth Factors |
|
|
|
What are the clinical signs of Inflammation?
|
Heat
Pain Redness Swelling |
|
|
|
What serum proteins accumulate in the tissue during inflammatory response?
|
Complement Proteins
Antibodies Cytokines Chemokines Growth Factors Albumin |
|
|
|
Which innate immune cell generates dif. kinds of pro-inflammatory cytokines?
|
Macrophage = one of the 1st cells on the scene (like channel one news...)
Produces a lot of inflammatory cytokines |
|
|
|
Why is fever good for us?
|
Virus/Microbes don't replicate well at high temperatures, but our immune cells DO work well at high temps.
Immune cells like it HOT. |
|
|
|
What are the actions of Macrophage-derived cytokines TNF-alpha, IL-1, IL-6? (=occur during inflammation)
|
Liver - Acute-phase proteins, like C-reactive protein, mannose-binding lectin --> Activation of Complement Opsonization
Bone Marrow/Endothelium - Nuetrophils --> Phagocytosis Hypothalamus - Inc. Body Temp. --> Dec. Viral and Bacterial Replication Fat/Muscle - Protein/Energy mobilization --> Inc. Body Temp. --> Dec. Viral and bacterial replication |
|
|
|
What proteins are part of the Acute-Phase Response?
|
Protein C (CRP, Complement Activation) and MLB (Initiates Lectin Complement pathway)
Goal: Get rid of infection |
|
|
|
Bacteria induce macrophages to produce what?
|
IL-6, which acts on hepatocytes to induce synthesis of acute-phase proteins like mannose-binding lectin, fibrinogen, and C-reactive protein
|
|
|
|
What does C-Reactive Protein do?
|
Binds phosphocholine on bacterial surface acting as opsonin and a complement activator
|
|
|
|
What does mannose-binding lectin do?
|
Mannose-binding lectin binds carbs carbs on bacterial cell surface acting as opsonin and complement activator
|
|
|
|
When they sense microbial products, what pro-inflammatory cytokines do macrophages secrete?
|
IL-6
TNF-alpha IL-1beata CXCL8 (=IL-8) IL-12 ALL Increase permeability of vasculature so things can get to an area of infection |
|
|
|
What does IL-12 do?
|
Inc. Th1 cells
Dec. Th2 cells |
|
|
|
What does IL-8 (=CXCL8) do?
|
Recruits Neutrophils and Basophils to site of infection
|
|
|
|
What does IL-1beta do?
|
Activates:
Vascular Endothelium Lymphocytes Local Tissue Destruction Increases Access of Effector Cells Systemic: Fever Production of IL-6 |
|
|
|
What does TNF-alpha do?
|
Activates:
Vascular Endothelium Inc. Vascular Permeability = inc. entry of complement cells Inc. Fluid Drainage to Lymph Nodes Systemic: Fever Mobilization of Metabolites Shock |
|
|
|
What does IL-6 do?
|
Only Systemic:
Fever Induces Acute-Phase Protein Production by Hepatocytes |
|
|
|
Can inflammatory effects of cytokines be bad?
|
Yes
|
|
|
|
What is Sepsis?
|
Bacterial infection in blood resulting in toxic effects
Usually caused by GRAM NEGATIVE - bacteria |
|
|
|
What is Septic Shock?
|
Caused by systemic release of cytokine TNF-alpha after bacterial infection of bloodstream
Dec. blood volume, Hypoproteinemia, Neutropenia, Neutrophilia Dec. Blood Volume = Causes collapse of blood vessels and multi-organ failure Frequently Fatal |
|
|
|
What innate molecules in our bodies causes Septic Shock
|
TNF-alpha (=released by macrophages)
Can lead to death when released systemically |
|
|
|
What are two Type 1 Interferons
|
IFN-alpha
IFN-beta |
|
|
|
What does IFN-alpha do?
|
Induces antiviral response and increases MHC class I expression on surface of infected cells
Produced by Leukocytes and Dendritic Cells |
|
|
|
What does IFN-beta do?
|
Same as IFN-alpha: Induces antiviral response and increases MHC class I expression on surface of infected cells
Produced by Fibroblasts and Virus-infected cells |
|
|
|
What is the major Type 1 Interferon Producing cell in the body?
|
Plasmacytoid Dendritic Cell
=Good at detecting viruses in blood and initiating the appropriate adaptive immune response |
|
|
|
Detection of PAMPs stimulates what?
|
Production of Type 1 Interferons (INF-alpha and IFN-beta - can shut down viral infection)
|
|
|
|
What are the actions of Type 1 Interferons (=INF-alpha and INF-beta)?
|
1. Induce resistance to viral replication in all cells (neighboring cells)
2. Inc. expression of ligands for receptors on NK cells 3. Activate NK cells to kill virus-infected cells |
|
|
|
What are the Pro-inflammatory Cytokines? The Ant-Viral cytokines? Adaptive Immune Cell Cytokines?
|
Pro-inflammatiory Cytokines = IL-1, IL-6, IL-8, IL-12, TNF-alpha
Anti-Viral Cytokines = Type 1 Interferons IFN-alpha and IFN-beta Immune Cell Cytokines = IL-2, IFN-gamma |
|
|
|
How does an Antigen Presenting cell drive differentiation of CD4+ cells into Th1, Th2, Th17, Tregs?
|
Antigen Presenting Cells produce cytokines that drive differentiation of CD4+ cells into Th1, Th2, Th17 and Tregs
|
|
|
|
What does the Adaptive immune cell cytokine IL-2 do?
|
Activation and proliferation of T cells
|
|
|
|
What does the Adaptive immune cell cytokine IFN-gamma do?
|
Activates macrophages, NK cells, Neutrophils and Dendritic Cells
|
|
|
|
When an antigen presenting cell produces IL-12, what does that induce Th1 cells to do?
|
Makes Th1 Cells Produce IL-2 and IFN-gamma which promote T Cell Proliferation and Macrophage activation
|
|
|
|
When an Antigen producing cell produces IL-4, what does that induce Th2 cells to do?
|
Makes Th2 cells produce IL-4, IL-5, IL-6, IL-10, IL-13
= INC. Humoral Immunity = Eosinophil-Mediated Destruction of Pathogens |
|
|
|
T/F: CD40L (=CD40 Ligand) on Th1 cells helps to activate macrohages resulting in enhanced killing
|
TRUE
|
|
|
|
What does CD40L on Th2 cells help to do?
|
Activate B Cells
|
|
|
|
When an Antigen producing cell produces IL-6 and TGF-beta, what does that induce Th17 cells to do?
|
Recruit Neutrophils
Inc. Inflammation NOTE: BOTH IL-6 AND TGF-beta have to be present to Induce Th17 cells |
|
|
|
Without the presence of IL-6, what does TGF-beta alone do when produced by antigen presenting cells?
|
Makes Treg produce IL-10 and TGF-beta which suppress adaptive immune responses
|
|
|
|
T/F: TGF-beta in ABSENCE of IL-6 will give us a regular T cell (Treg)
|
TRUE
|
|
|
|
What are the two major immunosuppressive and anti-inflammatory cytokines?
|
TGF-beta and IL-10
|
|
|
|
What does TGF-beta do?
|
Suppresses antigen-specific Th1 and Th2 cells
Blocks dendritic maturation Induces isotype switching to IgA Role in generating Tregs and is produced by Tregs |
|
|
|
What does IL-10 do?
|
Blocks differentiation of Th2 cells
Inhibits IFN-gamma and IL-2 production Inhibits IL-4 and IL-5 production from Th2 cells Inhibits Inflammatory cytokine production from phagocytes |
|
|
|
List some cytokines that induce isotype switching
|
IFN-gamma = induces switching to IgG1
IL-4 and IL-3 Induce switching to IgE (activates mast cells ie role in inflammation) TGF-beta induces switching to IgA (mucosal immunity) |
|
|
|
Hematopeiesis is the generation of?
|
Cells of the blood including RBCs, WBCs and Platelets
|
|
|
|
T/F: RBCs, WBCs, and platelets all derive from pluripotent hematopoietic stem cells
|
TRUE
|
|
|
|
What is the hematopoeitic stem cell marker?
|
CD34
|
|
|
|
What are the hematopoetic cytokines?
|
G-CSF
GM-CSF |
|
|
|
What does the hematopoeitic stem cell marker G-CSF do?
|
Promote proliferation of granulocyte precursors (neutrophils, eosinophils and basophils)
|
|
|
|
What does the hematopoeitic stem cell marker GM-CSF do?
|
Growth and differentiation of dendritic cells, monocytes, macrophages, granulocytes
|
|

