![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
Functional Group Nomenclature |
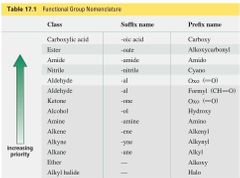
|
|
|
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR A KETONE WITH A GRIGNARD REAGENT |
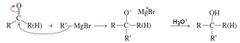
|
|
|
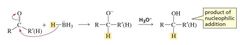
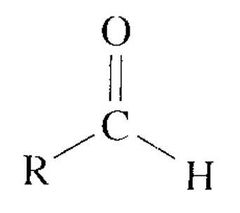
What type of reaction occurs between an aldehyde/ketone and a Gringard reagent? |
Aldehydes and ketones undergo nucleophilic addition reactions with Grignard reagents |
|
|
What is a Gringard Reagent? |
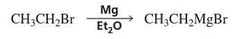
Gringard reactions with aldehydes/ketones often incur the formation of C-C and O-H bonds, and the breaking of a C-O bond.
Mg + Et20 |
|
|
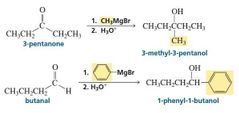
Examples of aldehyde/ketone reactions with Gringard Reagent |
|
|
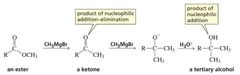
MECHANISM FOR THE REACTION OF AN ESTER WITH A GRIGNARD REAGENT |
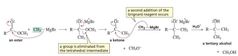
|
|
|
Why doesn’t a Grignard reagent add to the carbonyl carbon of a carboxylic acid? |

We know that Grignard reagents add to carbonyl carbons, so if we find that a Grignard reagent does not add to the carbonyl carbon, we can conclude that it must react more rapidly with another part of the molecule. A carboxylic acid has an acidic proton that reacts rapidly with the Grignard reagent, converting it to an alkane. |
|
|
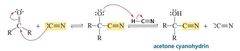
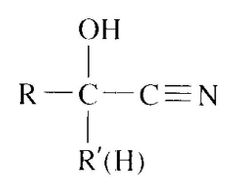
What is a Cyanohydrin? |
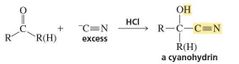
Cyanide ion is another carbon nucleophile that can add to an aldehyde or a ketone. The product of the reaction is a cyanohydrin . |
|
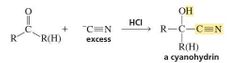
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR KETONE WITH HYDROGEN CYANIDE |
|
|
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR A KETONE WITH HYDRIDE ION |
|
|
MECHANISM FOR THE REACTION OF AN ACYL CHLORIDE WITH HYDRIDE ION |
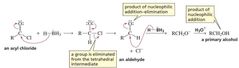
|
|
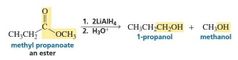
MECHANISM FOR THE REACTION OF AN ESTER WITH HYDRIDE ION |
|
|
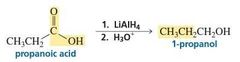
MECHANISM FOR THE REACTION OF A CARBOXYLIC ACID WITH HYDRIDE ION |
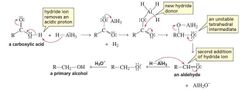
|
|
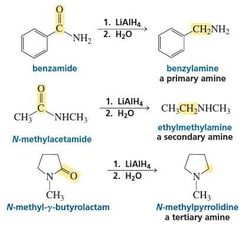
MECHANISM FOR THE REACTION OF AN N -SUBSTITUTED AMIDE WITH HYDRIDE ION |
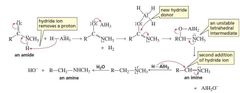
|
|
|
components of H:H |

|
|
|
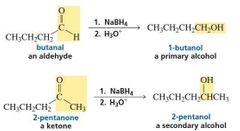
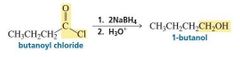
Reduction by Addition of a Hydride Ion and a Proton |
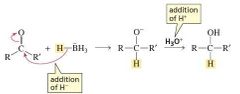
When aldehydes and ketones are reduced to alcohols by NaBH 4 , we have seen that the reduction occurs as a result of the addition of a hydride ion followed by the addition of a proton. |
|
|
Reduction by Addition of Two Hydrogen Atoms |
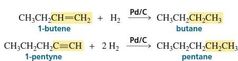
catalytic hydrogenations, the H-H bond breaks homolytically, so reduction results from the addition of two hydrogen atoms to the reactant. |
|
|
catalytic hydrogenations - carbon–nitrogen double and triple bonds |

|
|
|
catalytic hydrogenations - C=O |
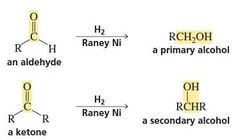
|
|
|
|
|

|
|
|

|
|
|
|
relative reactivities of carbonyl compounds |

|
|
|
HOW ALDEHYDES AND KETONES REACT |
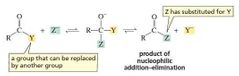
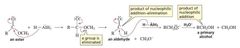
nucleophilic addition–eliminationreactions - Carboxylic acid derivatives undergo nucleophilic addition–elimination reactions with nucleophiles.
the nucleophile adds to the carbonyl carbon and a group is eliminated from the tetrahedral intermediate. Overall, it is a substitution reaction in which Z − substitutes for Y − . |
|
|
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR A KETONE WITH A GRIGNARD REAGENT - Formaldehyde |

When a Grignard reagent reacts with formaldehyde, the product of the nucleophilic addition reaction is a primary alcohol. |
|
|
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR A KETONE WITH A GRIGNARD REAGENT - Aldehyde |

When a Grignard reagent reacts with an aldehyde other than formaldehyde, the product of the nucleophilic addition reaction is a secondary alcohol . |
|
|
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR A KETONE WITH A GRIGNARD REAGENT - Ketone |

When a Grignard reagent reacts with a ketone, the product of the nucleophilic addition reaction is a tertiary alcohol . |
|
|
MECHANISM FOR THE REACTION OF AN ALDEHYDE OR A KETONE WITH A GRIGNARD REAGENT - Carbon Dioxide |
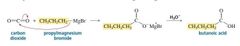
A Grignard reagent can also react with carbon dioxide. The product of the reaction is a carboxylic acid that has one more carbon than the Grignard reagent. |
|
|
What is an Acetylide Ion |

We have seen that a terminal alkyne can be converted into an acetylide ion by a strong base. |
|
|
THE REACTIONS OF CARBONYL COMPOUNDS WITH ACETYLIDE IONS |
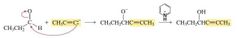
An acetylide ion is another example of a carbon nucleophile that reacts with an aldehyde or a ketone to form a nucleophilic addition product. When the reaction is over, a weak acid (one that will not react with the triple bond, such as the pyridinium ion shown here) is added to the reaction mixture to protonate the alkoxide ion. |
|
|
Cyanohydrin in basic solution |
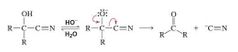
Cyanohydrins, however, are stable. The OH group will not eliminate the cyano group because the transition state for the elimination reaction would have a partial positivecharge on the oxygen, thereby making it relatively unstable. If, however, the OH group loses its proton, then the cyano group will be eliminated because the oxygen atom would then have a partial negative charge in the transition state. Therefore, in basic solutions, a cyanohydrin is converted back to the carbonyl compound. |
|
|
Usefulness of Cyanohydrins |
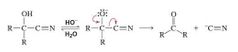
The addition of hydrogen cyanide to aldehydes and ketones is a synthetically useful reaction because of the subsequent reactions that can be carried out on the cyanohydrin. For example, the acid-catalyzed hydrolysis of a cyanohydrin forms an a-hydroxycarbox-ylic acid |

