![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
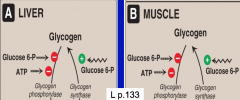
How does glycogen degradation differ in liver and muscle?
|
In the liver, glycogen is regulated by hormones. Takes place during fasting at low insulin/glucagon ratio.
RELEASE glucose into blood. In muscle, glycogen degradation is linked to muscle contraction, independent of hormones. Glucose 6-P is used in glycolysis (MUSCLE KEEPS IT FOR OWN METABOLISM). |
|
|
What is the affect of epinephrine on glycogenolysis?
|
Hormone stimulates glycohenlysis in both muscle and liver.
|
|
|
When does glycogen degradation, glycolysis and gluconeogenesis take place in liver and muscle?
|
In liver, glycogen degradation and gluconeogenesis take place at the same time at low insulin/glucagon. The liver glycogen is depleted after about one day.
In muscle, glycogen degradation and glycolysis taken place at the same time during muscle contraction. Muscle glycogen store is not depleted during fasting but during extensive extensive muscle exercise. |
|
|
Where in the human body is glycogen mainly found?
|
Overall largest amount is in skeletal muscle.
Highest concentration is in liver (10%). |
|
|
What is the purpose of glycogen metabolism?
|
- It is synthesized when glucose 6-P is abundant (high blood insulin levels).
- It is degraded when when glucose 6-P is needed (low insulin/glucagon ratio in liver/muscle contraction). |
|
|
In which cellular compartment is glycogen found and metabolized?
|
Glycogen and its enzymes for degradation and synthesis are both in cytosol.
Exception - also found in lysosomes |
|
|
What is the basic structure of glycogen?
|
It is a storage polysaccharide made up of glucose units that are linked by α1→4 glycosidic linkages and branch at α1→6 linkages.
|
|
|
How is UDP-glucose synthesized and what is it used for?
|
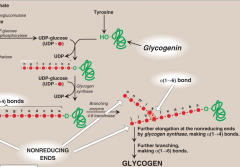
Glucose 6-P is converted to Glucose 1-P by enzyme phosphoglucomutase. UDP-glucose + PPi are then synthesized from Glucose 1-P by UDP-glucose pyrophosphorylase. UDP-glucose is a highly activated form of glucose for synthesis of glycogen.
|
|
|
What is the function of glycogen synthase and what is glycogenin?
|
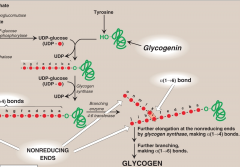
It is a self-glycosylating enzyme
It is responsible for making the α1→4 linkages in glycogen. It cannot synthesize free glucose but can only elongate existing chains of glucose. - A fragment of glycogen whose store are not totally depleted serves as a primer. In absence of this, a protein glycogenic can serve as acceptor for UDP-glucose and hydroxyl unit of a specific tyrosine serves as initial site where glycogenic catalyzes transfer of a few molecules of glucose to produce a short α1→4 chain (8 linkages) -This will serve as a primer for glycogen synthase. |
|
|
What is the function of 4:6 transferase?
|
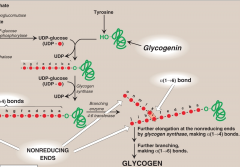
It is the branching enzyme that further elongates glycogen during synthesis.
It removes a chain of 6-8 glycosyl residues from the non-reducing end by cleavage of α1→4 bonds and attaches non-terminal glycosyl residues by a α1→6 linkage. |
|
|
Explain the hormonal regulation of glycogen synthase?
|
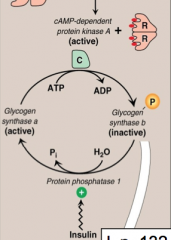
Glucagon and epinephrine bind to specific G-protein coupled receptor which results in synthesis of cAMP which activates cAMP dependent PROTEIN KINASE A. Protein kinase A (PKA) phosphorylates glycogen synthase (B) rendering it inactive (glycogen synthesis is inhibited).
High Insulin levels activate protein phosphatase-1, which cleaves phosphate from glycogen synthase (A) making it active (glycogen synthesis is on) |
|
|
What is function of glycogen phosphorylase? and PLP
|
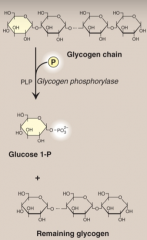
It is cystolic enzyme that degrades glycogen by cleaving at α1→4 bonds and produces Glucose 1-P. Pyridoxal phosphate (PLP) is a coenzyme require by glycogen phosphorylase.
PLP is formed from Vitamin B6. |
|
|
What is the advantage of glycogenolysis in anerobic glycolysis?
|
Usage of glycogenolysis as a results of glucose phosphorylase results in phosphorylated glucose. This saves one ATP that would be required during glycolysis (hexokinase). This results in net gain of 3-ATP compared to 2 ATP.
|
|
What is the allosteric regulation of glycogen synthesis and degradation in muscle and liver?
|
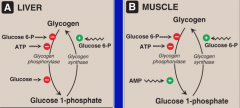
In both liver and muscle:
--Glucose synthase is (+) activated by Glucose 6-P --Glucose phoshporylase is (-) by Glucose 6-P and ATP In Liver: Glucose inhibits glycogen phosphorylase In Muscle: AMP stimulates glucose phosphorylase |
|
|
What is a limit dextrin during glycogenolysis?
|
Glycogen phosphorylase cleaves glucosyl residues from glycogen until four glucosyl residues remain on each chain before a branch point. The resulting structure is called a limit dextrin.
|
|
|
What is the debranching enzyme?
|
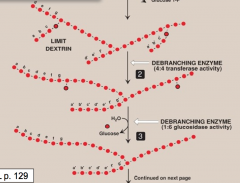
It is a bifunctional enzyme that removes branches during glycogenolysis.
The first activity is of 4:4 transferase removes the outer 3 out of the 4 residues on limit dextrin and transfers to non-reducing end of another chain. The remaining single residue linkage is cleaved by 1:6 glycosidase activity, releasing one free glucose. |
|
|
How is activation of glycogen done by cAMP-directed pathway?
|
Glycogen phosphorylase exists in two forms (a) - active and (b)- inactive form. Active PKA (cAMP dependent - activated by epinephrine or glucagon) phosphorylates the inactive (b) form to the (a) of glycogen phosphorylase kinase. This phosphate can be removed by insulin which produce protein-phosphatase -1.
Glycogen phoshprylase kinase (a) phosphorylates glycogen phosphorylase (b) to its active form. When active it will degrade glycogen. Insulin can cleave the phosphate via protein-phosphatases-1 to (b) form. |
|
|
How does calcium activate glycogen degradation?
|
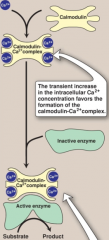
Ca+ is released into the cytoplasm by muscle during contraction or in liver by epinephrine. Ca binds to calmodulin, which allows 4 Ca2+ ions to bind. This triggers a conformation change to activate Ca2+-calmodulin.
Calmodulin can then activate various proteins such as glycogen phoshorylase kinase (b) to its active form with PKA. This can then activate glycogen phosphorylase which leads to glycogen degradation. |
|
|
How does AMP activate glycogen synthesis?
|
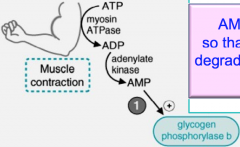
Muscle during extreme conditions, AMP increases which binds and allosterically activates glycogen phosphorylase b, without it being phosphorylated.
Remember that AMP also activates PFK-1 so that glycolysis can begin. |
|
|
What is Von Gierke Disease (Type 1 GSD)? How common is it, and what is affected?
|
25% - affects liver and kidney
1a: deficiency of glucose 6-phosphatase in the ER membrane 1b: deficiency of glucose 6-P translocase Both glycogen degradation and gluconeogenesis is impaired - results in severe hypoglycemia - Hepatonephromegaly - high glycogen content in liver + kidney -Severe fasting hypoglycemia (low blood glucose -Lactic Acidemia -Hyperuricemia -Hyperlipidemia -Progressive Renal failure |
|
|
What is Pompe Disease (Type II GSD)?
|
15% - Heart, liver muscle
Defective glycogen degradation in lysosomes by acid maltase (α1→4 glucosidase) - Results in lysosomal glycogen accumulation - which can rupture. -Blood glucose is normal and normal glycogen Affects: Massive cardiomegaly, myopathy, hypotonia, hepatomegaly Early death in first year - infantile Treatable by enzyme infusion |
|
|
What is Cori Disease (Type III GSD)
|
24% - Heart, Muscle nad Liver
Deficiency in the debranching enzyme (Forbes disease or limit dextrinosis) Results in high amount of glycogen in liver, heart and muscle --> results in abnormal glycogen structure with short branches - accumulation of limit dextrinios (usually an intermediate) Affects: Muscle weakness, hypotonia cardiomyopathy Muscle - muscular dystrophy Liver - mild hypoglycemia Hepatomegaly |
|
|
What is Anderson Disease (Type IV GSD)
|
3%
Deficiency of glycogen branching enzyme during synthesis . Results in abnormal glycogen structure with long glucose chains with less branches. - low amount of glycogen in liver and muscle Affects: Liver: infantile cirrhosis Muscle: infantile hypotonia Early death - by 5 years |
|
|
What is McArdle Syndrome (Type V GSD)?
|
2%
-Caused by deficiency of muscle phoshporylase - reduced and abnormal glycogen degradation in muscle - Will have normal blood glucose because liver - isozyme is normal Results in high amount of glycogen in muscle Causes: Muscle weakness and cramping Rhabdomyolysis - due to lack of ATP - serum CK-MM increase - test for no increase of lactate after exercise |
|
|
What is Hers disease (Type VI GSD)?
|
30%
Deficiency of hepatophosphorylase, muscle isozyme is normal - results in abnormal glycogen degradation in the liver. Will have high amount of glycogen only in muscle. Results in mild-fasting hypoglycemia Liver only : mild hypoglycemia |
|
|
What is Tarui Disease (VII GSD)?
|
0.2% - very rare
Is the deficiency of PFK-1 - reduced activity - in muscle (MM) and RBC. Results in muscle cramping due to lack of ATP. Have high glycogen amount in muscle. HEMOLYSIS in RBC - lack of ATP Normal blood glucose |

