![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
isotopes
|
same atomic number different mass number because different number of neutrons
|
|
|
speed of an atom (ex: He) compared to another atom (ex: Ar). |
speed of He / speed of Ar = sqr root of MW of Ar / sqr root of MW of He |
|
|
total deltaH of a reaction |
deltaH of products - deltaH of reactents. include every product and reactant including solids. |
|
|
u r told a compound has 40% Carbon, 6.6% Hydrogen, and 53.3% Oxygen. u r asked to find the emperical formula of the compound. what are the steps. |
1. write the percentages : C40H6.6O53.3 2. divide by mass of each: C 40/12 H 6.6/1 O 53.3/16 3. divide by smallest number : C 3.3/3.3 H6.6/3.3 O 3.3/3.3 4. what u get is ur emperical formula. |
|
|
when there is a pinhole what gas escapes fastest |
the lightest gas and it exerts the least amount of pressure. |
|
|
number 226 |
page 175. its suppose to be easy |
|
|
critical point |
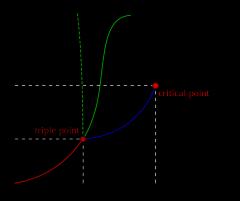
final point on the vapor pressure curve
from left it goes solid/liquid/vapor |
|
|
reactions of Na, Li, and K when reacted with excess H2O |
for example Na: NaOH + H2 u always end up getting H2 |
|
|
how to find formal charge on an atom in a molecule. |
Formal charge = ve - [1/2 (# shared e) + # unshared e] |
|
|
when does gas best dissolve in a solution? |
under conditions of high pressure and low temperature. these increase gas solubility |
|
|
actual mass of an atomic nucleus |
always less than the sum of the protons and nutrons. this mass difference is called the mass defect. this also shows the binding energy which is the energy to form the nucleus. the larger the binding energy the larger the energy needed to break it and the more stable the nucleus is. |
|
|
heating a liquid |
as temp is increased, vapor pressure would increase but viscosity and surface tension would decrease. |
|
|
viscosity |
The viscosity of a fluid is a measure of its resistance to gradual deformation by shear stress or tensile stress. the lower the viscosity the easier to boil and evaporate |
|
|
surface tension |
is a contractive tendency of the surface of a liquid that allows it to resist an external force. |
|
|
vapor pressure |
high vapor pressure means low boiling point. it depends on the intermolecular attractions of the substance. molecules that can h-bond have higher boiling point and lower vapor pressure. smaller molecules have less intermolecular attraction and have lower bp therefore higher vapor pressure. |
|
|
number 240 |
page 241. ask someone |

