![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
Arrhenius Acids
|
Dissociate to produce an excess of hydrogen ions in solution (H+)
|
|
|
Arrhenius Bases
|
Dissociate to produce an excess of hydroxide ions in solution (OH-)
|
|
|
Bronsted-Lowry Acids
|
Species that can donate hydrogen ions
|
|
|
Bronsted-Lowry Bases
|
Species that can accept hydrogen ions
|
|
|
Lewis Acids
|
Species that accept electrons
|
|
|
Lewis Bases
|
Species that donate electrons in lone pairs
|
|
|
Differences between Definitions
|
Arrhenius is most restrictive and limited to aqueous solutions, Bronsted focuses on H, Lewis focuses on lone pairs
|
|
|
Amphoteric Species
|
Can behave as an acid or base
|
|
|
Amphiprotic Species
|
Amphoteric species that specifically behave as Bronsted-Lowry acids or bases
|
|
|
Autoionization of Water
|

|
|
|
Water Dissociation Constant
|
Kw, is 10-14 at 298 K (25 C)
|
|
|
pH
|

|
|
|
pOH
|
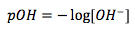
|
|
|
Relationship Between pH and pOH
|
As pH increases, pOH decreases so that the sum is always 14 at 298 K
|
|
|
Strong Acids and Basis
|
Completely dissociate in solutions
|
|
|
Common Strong Acids
|
HCl (hydrochloric acid), HBr (hydrobromic acid), HI (hydroiodic acid), H2SO4 (sulfuric acid), HNO3 (nitric acid), and HClO4 (perchloric acid)
|
|
|
Common Strong Bases
|
NaOH (sodium hydroxide), KOH (potassium hydroxide) and other soluble hydroxides of group IA metals
|
|
|
Converting Concentration to p Value
|
[x] = 10-n then pX = n
|
|
|
Estimate for Complicated Conversion to p Value
|

|
|
|
Weak Acids and Bases
|
Do not dissociate completely
|
|
|
Impact of Autoionization with Strong Acids and Bases
|
Only negligibale if concentration of acid or base is greater than 10^7
|
|
|
Acid Dissociation Constant
|
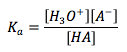
|
|
|
Base Dissociation Constant
|
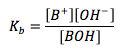
|
|
|
Dissociation Constant of Weak Acids and Basis
|
The smaller the constant the weaker the acid or base, must be less than 1.0
|
|
|
Strength of Conjugate
|
Strong acids and bases have weak (inert) conjugates while weak acids and bases also have weak conjugates
|
|
|
Induction
|
Acids with electronegative elements near an acidic proton have increased acidic strength
|
|
|
Neutralization Reactions
|
React acids and bases together to form salts (and sometimes water)
|
|
|
Combinations for Neutralization Reactions
|

|
|
|
Resulting pH of Neutralized Solution
|
Two strong: neutral, two weak: depends on relative strength of both, Strong acid: acidic, Strong base: basic
|
|
|
Equivalents
|
One mole of the species of interest (H+ for acids, OH- for bases)
|
|
|
Normality
|
The concentration of acid or base equivalents in solution equal to the multiplication of the concentration of the reactant by its number of equivalents
|
|
|
Polyvalent Acids and Bases
|
Can donate more than one equivalent
|
|
|
Titrations
|
Used to determine the concentration of a known reactant in a solution
|
|
|
Titrant
|
Has a known concentration and is added slowly to the titrand to reach the equivalence point
|
|
|
Titrand
|
Has an unknown concentration but a known volume
|
|
|
Half Equivalence Point
|
Midpoint of the buffering region in which half of the tritrant has been protonated or deprotonated
|
|
|
Equivalence Point
|
The steepest slope in a titration curve reached when the number of acid equivalents in the original solution equals the number of base equivalents added (or vice versa)
|
|
|
Equivalence Point Trends
|

|
|
|
Indicators
|
Weak acids or bases that display different colors in their protonated and deprotonated forms
|
|
|
Choosing an Indicator
|
Should have a pKa close to the pH of the expected equivalence point
|
|
|
Buffer Solutions
|
Mixture of a weak acid and its conjugate salt or a weak base and its conjugate salt and are used to resist large fluctuations in pH
|
|
|
Buffering Capacity
|
Ability to resist changes in pH, maximum within 1 pH point of the pKa of the acid in the solution
|
|
|
Henderson-Hasselbalch Equation- Weak Acid
|
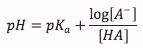
|
|
|
Henderson-Hasselbalch Equation- Weak Base
|

|
|
|
IdentifyingType of Titration |
Identify starting point If pH >> 7: titrant is a strong base If pH > 7: titrant is a weak base If pH < 7: titrant is weak acid If pH << 7: titrant is a strong acid |

