![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
Gases
|
Least dense form of matter Fluid and conform to the shape of their container Easily compressible
|
|
|
Variables to Describe Gases
|
Temperature, pressure, volume, number of moles
|
|
|
Standard Temperature and Pressure
|
273 K and 1 atm
|
|
|
Equations for Ideal Gases
|
Assume negligible volume and mass of gas molecules
|
|
|
Ideal Gas Law
|

|
|
|
Avogadro’s Principle
|
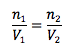
Pressure and temperature held constant
|
|
|
Boyle’s Law
|
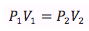
Temperature and number of moles held constant
|
|
|
Charles’s Law
|
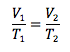
Pressure and number of moles held constant
|
|
|
Gay-Lussac’s Law
|
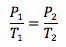
Volume and number of moles held constant
|
|
|
Combined Gas Law
|
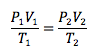
Combination of other gas laws
|
|
|
Density
|
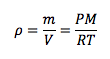
|
|
|
Dalton’s Law of Partial Pressure
|

|
|
|
Henry’s Law
|
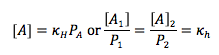
Amount of gas dissolved in solution is directlyproportional to the partial pressure of that gas at the surface |
|
|
Kinetic Molecular Theory Assumptions
|
Gas particles have negligible volume, lack intermolecular attractions and repulsions, have elastic collisions, average kinetic energy is directly proportional to temperature
|
|
|
Average Molecular Speeds
|

|
|
|
Rule of Thumb Gas Molecule Speed
|
Higher temp, faster the molecule
Larger molecule, slower the molecule |
|
|
Graham’s Law
|
Gases with lower molar masses will diffuse or effuse faster than gases with higher molar masses at the same temperature
|
|
|
Real Gases
|
Deviate from ideal behavior under high pressure (low volume) and low temperature conditions
|
|
|
Real Gases Occupy Less Volume
|
At moderately high pressures, low volumes, or low temperature because of intermolecular forces
|
|
|
Real Gases Occupy More Volume
|
At extremely high pressures, low volumes, or low temperatures because particles occupy physical space
|
|
|
Van der Waals Equation of States
|
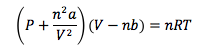
Corrects the ideal gas law for intermolecular forces (a) and molecular volume (b)
|

