![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
52 Cards in this Set
- Front
- Back
|
cell injury vs cell death
|
injury = reversible
death = not |
|
|
what cellular systems are vulnerable to injury
|
cell membrane
genetic apparatus energy supply proteins |
|
|
causes of cell membrane injury
|
trauma
free radicals weaponized pores |
|
|
sources of free radicals
|
radiation injury
toxicity inflammation - oxidative burst - nitric oxide normal cell fxn |
|
|
what causes weaponized pores
|
complement cascade
bacterial hemolysins |
|
|
what causes DNA injury
|
free radicals
viruses toxins radiation |
|
|
what injures cells by affecting energy supply
|
toxins
- ie aflatoxin inhibits FA oxidation hypoxia |
|
|
what causes protein injury
|
toxins
heat/life -> heat shock proteins |
|
|
heat shock proteins
|
produced in response to heat or stress
act intracellularly - recognize - bind - chaperone - refold - degrade (chaperone to lysosome) conserved throughout evolution some role in - autoimmune dz - chemotherapy - anoxia survival |
|
|
response to injury depends on
|
type of cell
nutrition previous injury |
|
|
what are the 1st cells affected by injury?
what other tissues are intermediately sensitive? very resistant? |
extremely sensitive
- neurons intermediate susceptibility - myocardium - hepatocytes - renal tubular epithelium slow to be injured/ die - fibroblasts - epidermis - skeletal muscle |
|
|
Give an example where response to injury depends on nutrition
|
white muscle dz
Vit E and Selenium deficiency - both are antioxidants - they are component of glutathion peroxidase |
|
|
true or false
That which does not kill us makes us stronger |
false...
just ask my poor neurons right now after looking at this stuff for so long... |
|
|
morphology of injury
|
fatty change
protein accumulation glycogen accumulation hydropic degeneration |
|
|
____ is the most clinically significant morphology of cellular injury
|
fatty change
|
|
|
mechanisms of fat accumulation in hepatocytes
|
excessive entry of fatty acids
- foie gras defective oxidation of fatty acids - anoxia - many toxins (such as aflatoxin) decreased apoprotein synthesis - protein malnutrition - less protein coming in makes fat not able to get out of cell defective secretion of lipoproteins - toxins such as alcohol |
|
|
hydropic degeneration
|
reversible cell swelling that follows ischemia
transient change that is rarely seen (exc in epithelium) cells enlarged with clear watery cytoplasm - identical to glycogen accumulation may reverse to normal fxn or progress to cell rupture & death |
|
|
what happens to free fatty acids in circulation normally
|
free fatty acids
intracellular fatty acids -> oxidation in mitochondria or -> lipid exports (cholesterol/ phospholipids/ triglycerides) + apoprotein ->lipoprotein -> leaves cell -> circulating lipoproteins |
|
|
feline hepatic lipidosis syndrome pathogenesis
|
fat cat stops eating
-> mobilization from large fat stores -> EXCESSIVE FA ENTER LIVER -> lipid processing is overwhelmed -> protein availability reduced by anorexia -> DECREASED LIPID EXPORT -> fatty change -> liver dysfxn -> icterus and anorexia -> fat yellow cat -> still not eating -> repeat cycle -> dead fat yellow cat |
|
|
ruminant fatty change
|
late preg or early lactation in over-conditioned animals
intake doesn't keep up with nutritional demand large fat stores mobilized -> fatty liver |
|
|
morphology of fatty change
|
grossly
- enlarged - tan-yellow - may float if severe - friable histologically - clear, round, discrete cytoplasmic vacuoles feline renal tubular epithelium normally contains cytoplasmic lipid (don't confuse this with fatty change) |
|
|
fatty infiltration
|
infiltration of adipose CELLS into non-adipose tissue
different from fatty change (intracellular accumulation of lipid in non-adipose cells) most common in heart and pancreas |
|
|
define free radical
how does it cause damage |
chemical with unpaired electron in outer orbit
extremely unstable/ reactive damages membrane lipids by lipid peroxidation |
|
|
what creates free radicals
|
radiation injury
- sunlight-induced skin damage toxicity - many toxins converted into free radicals - cigarette smoke - oxygen - carbon tetrachloride CCL4 - chemotherapeutic agents inflammation - killing by neutrophils and macrophages requires oxidatice burst - nitric oxide from endothelial and inflammatory cells is a free radical normal cell fxn produces free radicals from oxygen |
|
|
_____ is the most toxic free radical
|
OH-
|
|
|
what antioxidants are constantly needed to inactivate free radicals
|
Vitamin E/ Selenium
- selenium is a component of glutathione peroxidase albumin, ceruloplasmin, & transferrin - bind copper and iron |
|
|
know the normal cell fxn and how it results in free radicals
|
O2
=> oxidases in mitochondria, ER, peroxisomes, and cytoplasm -> O2- => superoxide dismutase -> H2O2 => ferrous iron & other transitional metals -> OH- => glutathione peroxidase -> H2O need Vit E/ selenium (selenium is part of glutathione peroxidase), and albumin/ceruloplasmin/transferrin to bind Cu and Fe |
|
|
what causes DNA injury
|
free radicals
- react with thiamine to produce single strand breaks viruses - can insert into host cell genome toxins - aflatoxin binds to DNA forming adducts radiation - UV light forms thymine dimers |
|
|
what interferes with energy supply
|
toxins
- aflatoxin inhibits FA oxidation Hypoxia - very impt! - leads to cell death |
|
|
What inhibits protein synthesis/ damages proteins themselves
|
toxins
- amanita mushroom toxin (inhibits RNA polymerase) - ricin (inhibits ribosomes) Heat (and life in general) - denatures proteins |
|
|
what are the characteristics of heat shock proteins (3)
|
produced in response to cell stress (heat/ anoxia/ viral infections/ toxins)
act intracellularly to recognize/ bind/ chaperone/ refold/ degrade proteins conserved throughout evolution |
|
|
what is the medical relevance of heat shock proteins
|
autoimmune diseases often target HSP
HSP expression linked to resistance to anti-cancer drugs increased HSP expression related to survival of anoxia |
|
|
how does aflatoxin affect cells
|
inhibits fatty acid oxidation (results in fatty change)
binds to DNA forming adducts |
|
|
what causes defective fatty acid oxidation
|
anoxia
many toxins (such as aflatoxin) |
|
|
where is fatty infiltration most common
|
heart and pancreas
|
|
|
when do you see glycogen accumulation in dogs
|
steroid hepatopathy
|
|
|
pathogenesis of canine steroid hepatopathy
|
excess endogenous or exogenous glucocorticoids
-> induction of glycogen synthetase enzyme -> excess glycogen is produced and accumulates in hepatocyte cytoplasm -> hepatocyte dysfunction and elevated liver enzymes (esp ALP) |
|
|
know normal fatty acid processing
|
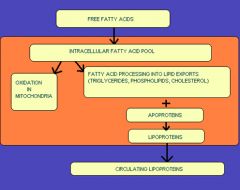
|
|
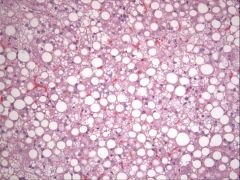
|
fatty change
|
|
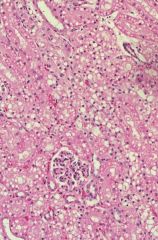
|
cat kidney
|
|
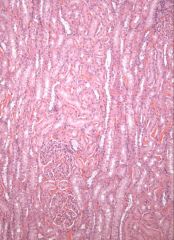
|
dog kidney
|
|
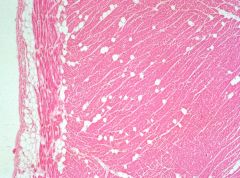
|
fatty infiltrate
Adipocytes move from epicardium into the heart (usually in R ventricle) (not fatty change b/c it adipocytes infiltrating, not lipid in cardiac cells) |
|
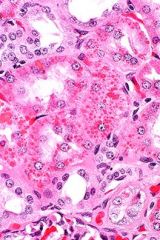
|
protein accumulation
rare eosinophilic cytoplasmic droplets not impt in vet med |
|
|
what does canine steroid hepatopathy look like
what is the prognosis? |
Grossly
- liver is enlarged, orange-brown, and friable Microscopically - midzonal hepatocytes are swollen with cleared prognosis - good |
|
|
causes of glycogen accumulation
|
canine steroid hepatopathy
diabetes mellitus storage diseases neonatal animals have abundant glycogen in hepatocytes normally |
|
|
why hepatocyte change is seen in patients with diabetes mellitus
|
glycogen accumulation
often see fatty change as well |
|
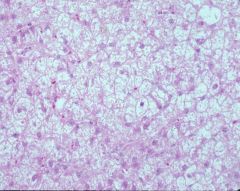
|
steroid hepatopathy
Pink hepatocytes normal, white ones are the affected ones (glycogen, like fat, is lost in processing) Zonal pattern Hazy/ lacy palllor clearing to cytoplasm No sinusoids b/c cells too swollen that they closed the sinusoids off Glycogen usually doesn’t displace the nucleus whereas the lipid usually does Prognosis good b/c injury |
|
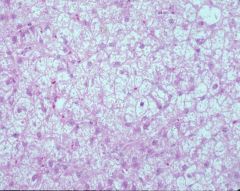
|
steroid hepatopathy
Pink hepatocytes normal, white ones are the affected ones (glycogen, like fat, is lost in processing) Zonal pattern Hazy/ lacy palllor clearing to cytoplasm No sinusoids b/c cells too swollen that they closed the sinusoids off Glycogen usually doesn’t displace the nucleus whereas the lipid usually does Prognosis good b/c injury |
|
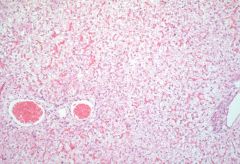
|
glycogen storage disorder
Congenital enzyme deficiency One of those can result in glycogen accumulation More diffuse Glycogen found in more cells than just hepatocytes |
|
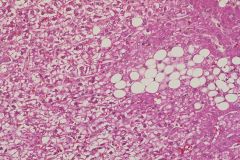
|
diabetes mellitus (glycogen accumulation and fatty change)
Still polygonal cells with nucleus in center Diabetes can produce both glycogen and fat accumulation in liver |
|
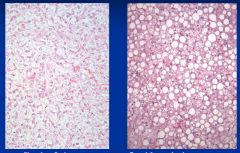
|
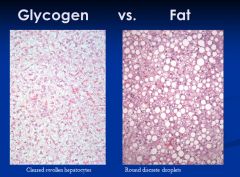
|
|
|
how do aflatoxins cause fatty change?
a. Excess entry b. Defective oxidation c. Decreased apoproteins d. Defective lipoprotein secretion |
b. Defective oxidation
(toxins can also affect lipoprotein secretion but aflatoxin interferes with oxidation) |

