![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
1. Be able to draw the structure of tyrosine, levodopa, and dopamine.
|
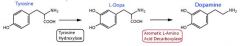
|
|
|
2. Describe the routes of metabolism for dopamine (this should involve the enzymes involved and the metabolic products) Hint: remembering the metabolites is easier if you know the structure of dopamine and what the enzymes do.
|
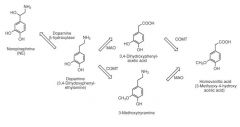
1. MAO oxidizes the carbon attached to the amine (CH2CH2-NH2) to a carboxylic acid (CH2-COOH)
2. COMT with SAM (S-adenylyl methionone) – methylates one of the –Ohs (closest to amine) – minor route 3. Dopamine B-hydroxylase hydroxylates the first CH2 of the amine chain NE |
|
|
3. Describe how the metabolism of dopamine might be involved in Parkinsonism pathogenesis.
|
1. Metabolism by MAO generates hydrogen peroxide, which in the presence of Fe2+, can generate superoxide and hydroxyl radical. The hydroxyl radical may contribute to the degredation of dopaminergic neurons = parkinsons.
|
|
|
4. Identify the MAO enzyme that should be preferentially inhibited for Parkinsonism treatment.
|
1. MAO-B, which has greater activity towards dopamine, should be preferentially inhibited for Parkinsonism treatment (where we want to increase DA levels)
|
|
|
5. Describe the metabolism of SELEGILINE and be able to recognize structures of the metabolites.
|
Selegiline (alkyne and methyl attached to amino end, Me replaces COOH) is metabolized by 2B6/2C18 (N-dalkylation = loss alkyne) methamphetamine undergoes a second demthylation = loss methyl by 2B6 / 2C19 to make amphetamine:
|
|
|
5. Describe the metabolism of SELEGILINE and be able to recognize structures of the metabolites.
|
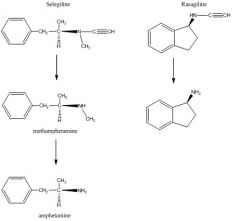
Selegiline (alkyne and methyl attached to amino end, Me replaces COOH) is metabolized by 2B6/2C18 (N-dalkylation = loss alkyne) methamphetamine undergoes a second demthylation = loss methyl by 2B6 / 2C19 to make amphetamine:
|
|
|
6. Provide a metabolism based explanation to explain why administration of rasagiline may result in decreased risk of vasoactive and/or hallucination side effects when compared to selegiline.
|
1. Selegiline is metabolized to a CNS stimulant (AMPHETAMINE) which can result in vasoconstriction and hallucinations, but Rasagiline is metabolized by CYP1A2 not a potent CNS stimulant.
|
|
|
7. Be able to tell a patient what L-DOPA stands for.
|
1. L-DOPA stands for Levodopa which is a precursor to Dopamine
|
|
|
8. Explain how vitamin B6 (pyridoxine) impacts L-DOPA treatment.
|
1. Pyridoxine (B6) is a cofactor for aromatic L-amino acid decarboxylase which metabolizes L-Dopa to dopamine. There is more LAAD in the periphery than in the brain, so if you increase B6 intake, you increase the amount of L-DOPA metabolized to Dopamine in the periphery.
If metabolized in the periphery, dopamine is ineffective in the brain (as it cannot pass through the BBB). And instead will increase dopaminergic side effects in the periphery via peripheral chemoreceptors (emetic effects). |
|
|
9. Identify the structural similarities and differences between levodopa and carbidopa.
|
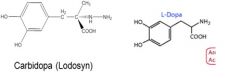
1. Caridopa has an additional methyl group, and amine group (creating a hydrazine) on the end of the molecule.
|
|
|
11. Describe the differences in duration of action and location of action for tolcapone and entacapone.
|
1. Tolcapone (Tasmar) – fulminant hepatic failure (last line tx)
1. Acts in the brain and the periphery as a reversible inhibitor of COMT 2. Longer duration of action (8-12 hr ½ life) 2. Entacapone (Comatan) 1. Acts mostly in the periphery 2. Shorter duration of action (2 hr ½ life) |
|
|
12. Explain why entacapone is used more widely than tolcapone.
|
1. Tolcapone is associated with fulminate (rapid onset) hepatic failure – a mechanism for toxicity is related to the nitrogroup hanging off of the benzene ring. – Entacapone does not have hepatic toxicity.
|
|
|
13. Describe a patient to whom tolcapone would be recommended.
|
1. If a patient has exhausted other drug options, then tolcapone could be used, but not before then.
|
|
|
16. Explain why some think that dopamine receptor agonists might be preferred as initial monotherapy rather than Sinemet (L-DOPA/carbidopa).
|
Dopamine oxidation in the presence of metals may result in the formation of toxic quinones. The quinones can bind to a nuclophile on a protein that can cause damage in the sustintia negra – which would increase parkinsonian symptoms.
|
|
|
14. Provide structural reasons to explain why AMANTADINE and MEMANTINE are able to penetrate the blood brain barrier. (adamanTADINE CAGE)
|
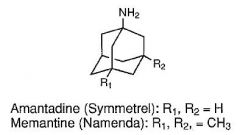
1. The cage-like hydrocarbon structure provides lipophilicity that likely assists in
penetration of the blood brain barrier |
|
|
18. Provide two reasons why donepezil might be preferred over tacrine for treating alzheimer’s disease.
|
1. Donepezil (Aricept)
1. little to no hepatoxicity; Tacrine has hepatoxicity T=toxic 2. much longer ½ life which allows 1x/day administration; Tacrine requires 4x/day – for patients with dementia this is a problem. |
|
|
15. Provide a chemical explanation that drives the hypothesis that dopamine is involved in the toxicity of dopaminergic neurons.
|
1. Metabolism by MAO generates hydrogen peroxide, which in the presence of Fe2+, can generate superoxide and hydroxyl radical. The hydroxyl radical may contribute to the degredation of dopaminergic neurons = parkinsons.
|

