![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
1. List the major sedative-hypnotic drug classes.
|
1. Barbiturates
2. Propanediol carbamates 3. Benzodiazepines 4. “Z drugs” (ALL start with Z except Lunesta (Eszopiclone) 5. Melatonin receptor agonist 6. Piperidinediones 7. Acetylene derivatives 8. Chloral hydrate 9. SSRIs (previous lecture) |
|
|
2. Describe the properties necessary for a barbiturate derivative to have good sedative-hypnotic activity.
|
1. Be a weak acid.
2. Have the appropriate lipid/water partition coefficient (log P). |
|
|
3. Define log P and explain what high and low values of log P mean.
|
1. It is a physical chemical property to estimate how these agents are going to act in the body. The log of the (concentration of the drug in octanol) / (concentration of the drug in water). Drug in octanol is acting as the lipophilic media.
2. High = lipophilic, low = hydrophilic |
|
|
4. Explain how log P is determined and what it can tell you about a drug.
|
2. If it is 100/2, you would say that log p = 2. The log p scale is 1-5 (x axis vs activity on y axis) and you look for an optimal log P. Log P is physical chemical quantitative measure of lipophilicity. Negative values would by hydrophilic. Positive values are lipophilic.
|
|
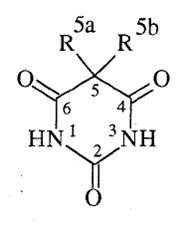
5. Explain how barbituric acid differs structurally from the barbiturate derivatives that are used as drugs.
|
1. Barbituric acid is too acidic to act as a sedative-hypnotic
1. The acid-base properties can be modified via substitution at the 5a and 5b positions. |
|
|
7. Explain how resonance stabilization impacts the acid/base properties of barbituric acid (draw the resonance structures).
|
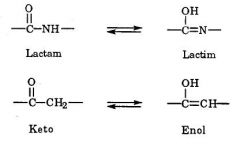
The acid-base properties of barbiturates are the result of lactam-lactim and ket-enol tautomerism
|
|
|
8. Describe how the pKa’s of barbituric acid derivatives are affected when substitutions are made at the 5 position and how this change affects pharmacology.
|
1. When 5a and 5b = Hydrogens, PKA = 4.1 (too acidic)
2. 5a, 5b = alkyl or other hydrocarbon containing groups = pKa of 7-8. By doing this you loose the keto-enol tautomerism. |
|
|
9. Given the pKa of a barbiturate, determine the ratio of unprotonated/protonated and ionized/unionized molecules at a given pH.
|
%I = 100/(1+10^(Ph-pKa)) IF a base, else swap ph/pka if acid
|
|
|
10. Describe the pharmacological effects observed when the nitrogen atoms of the barbiturates are modified. Provide a chemical explanation for these effects.
|
1. If we got rid of BOTH of the protons attached to the nitrogens, it will abolish activity (outside of the physiological PH range. We need at least one acidic proton. If you have both 5 positions and one of the nitrogens changed to an alkyl group – you still have activity.
|
|
|
11. Describe the effect of different alkyl group substitutions at the 5 position of the barbiturates.
|
1. Hypnotic activity increases with increasing lipophilicity until the total number of carbons at both the C-5 positions is between 6 and 10. Further increases lead to decreases in hypnotic activity.
2. Unsaturated allyl, alkenyl, and cycloalkenyl derivatives are more potent than the corresponding saturated analogs |
|
|
12. Describe the effect of incorporating stereochemistry into the 5 position of the barbiturates.
|
1. Branched chain isomers have greater lipid solubility and hypnotic activity than straight chains
2. Stereoisomers have the same potency (approximately). |
|
|
13. Describe the impact of modifications at the carbonyl carbons of the barbiturates.
|
1. The substitution of a sulfur atom for the oxygen at C-2:
1. Increases lipid solubility. 2. Produces quicker onset and short duration (thiobarbituates used for i.v. anesthesia). Substitution at more than one carbonyl oxygen with sulfur decreases hypnotic activity (i.e., there is an optimal lipophilicity range). |
|
|
14. Describe how the barbiturates are categorized and list the categories.
|
1. LONG = SEDATIVES
2. SHORT-INTERMED = HYPNOTICS 3. Ultrashort acting: i.v. ANESTHETICS |
|
|
15. Describe the factors that affect a barbiturate’s duration of action and the role that acidity/basicity and lipophilicity have in determining duration of action.
|
1. Renal Excretion
2. Metabolism 3. Redistribution (for short acting agents) That is, very little of the parent drug will be excreted unchanged in the urine. The causes are: Poor kidney filtration: Barbiturates are lipophilic, weakly acid drugs that are extensively bound to plasma proteins. Therefore, they are poorly filtered (i.e., they have to disassociate from the proteins in order to go into the urine). Extensive reabsorption: These are weakly acidic drugs and the urinary pH = 6. So the barbiturates are mostly unionized and, since they are also relatively lipophilic molecules, they are reabsorbed. |
|
|
16. Describe what could be done in cases of barbiturate overdose to eliminate the drug more rapidly. This involves predicting ratios of unionized to ionized drug at different pHs.
|
1. Sodium bicarbonate can be administered so that the urinary pH is increased. This increases the amount of drug existing in the ionized form, increases water solubility, and excretion (less reabsorption).
|
|
|
17. Explain why the duration of action of most barbiturates is determined by the rate of metabolism. Which type of barbiturates is an exception?
|
1. This is true for all BUT the ultrashort acting barbiturates.
2. Occurs because metabolism = more hydrophilic = higher rate of excretion. |
|
|
18. Describe the common metabolic routes of barbiturate metabolism and how the substituents at the C-5 position impact metabolism (e.g., what happens if R = an aromatic ring?).
|
Common metabolic routes include hydroxylation at 5-C or para position, followed by glucuronidation of hydroxylated metabolites
Aliphatic hydroxylation at one of the C-5 substituents (ω-1 hydroxylation) Aromatic hydroxylation is a relatively slow process for barbiturates compared to ω-1 hydroxylation. Therefore if this is the primary route of metabolism for a specific barbiturate then it will have a longer half-life. E.g., phenobarbital undergoes hydroxylation at the para position. Therefore it has a long half life (100 hours). Phenobarbital is a long acting sedative. Glucuronide conjugation of hydroxylated metabolites Some barbiturates also undergoes an unusual N-glucuronidation directly on the ring nitrogen. |
|
|
19. Describe the adverse events associated with the barbiturates.
|
2. Low Therapeutic Index
3. Tolerance and Dependence 4. Respiratory and Cardiovascular Depression |
|
|
20. Draw the benzodiazepine ring and label w/descriptions of the common structural groups found at R1, R2, and R3.
|
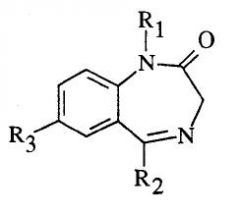
1. R1: Alkyl groups at this position are common but aren’t essential for activity.
2. R2: Most of the time these are aryl substituents. Ortho-substitution with electron-withdrawing groups (e.g., Cl or F) enhance potency. 3. R3: Electron withdrawing groups enhance potency. 4. Benzodiazepine structure can profoundly impact metabolism. This is important because rates of metabolism will impact DOA and therefore, how the particular benzodiazepine is used. |
|
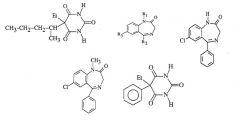
21. Given a set of structures, recognize a long-lived active metabolite of diazepam.
|
More lipophilic = short DOA but long elimination t ½ in lipid stores
|
|
|
22. Explain chemically why oxazepam and lorazepam can be administered as a racemate.
|
1. Equal activity, self racemizing
|
|
|
23. Describe and recognize the type of substitutions at the R1 position in the benzodiazepines that lead to long-lived active metabolites (that increase the effective half-life).
|
1. LONG = Alkyl group that is easily N-dealkylated
2. SHORT = 1. no alkyl group that’s easily N-dealk 2. –OH grp = easy glucuronidation = ez elimination |
|
|
24. Describe and recognize the type of substitutions at the R1 position in the benzodiazepines that lead to compounds that have relatively short effective half-lives.
|
1. SHORT ACTING a group at R1 that isn’t easily N-dealkylated
2. Shorter acting agents have structural features that allow them to be eliminated more quickly (e.g., triazole ring doesn’t allow formation of nordiazepam, α-hydroxy group allows rapid glucuronidation |
|
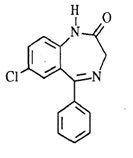
select the fastest metabolized drug(s) of the group and explain which feature is responsible and how it works.
|
Triazolam and Estazolam due to triazole ring, triazole ring doesn’t allow formation of nordiazepam, α-hydroxy group
allows rapid glucuronidation |
|
|
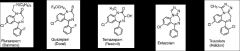
Given a set of molecules recognize the common metabolite of many benzodiazepines and explain why it is significant.
|
NORDIAZEPAM: long-lived metabolite resulting from
N-dealkylation = explains SAR for long t ½ |
|
|
29. Describe the metabolic processes that benzodiazepines undergo in order to generate the long lived active metabolite.
|
1. N-dealkylation
|
|
|
30. Describe how metabolism and duration of action can impact the selection of a benzodiazepine as a hypnotic (e.g., compare flurazepam metabolism to triazolam metabolism).
|
1. Flurazepam = long t ½ ez N-dealk
2. triazolam = short t ½ no N-dealk |
|
|
31. Identify “Z drugs” that are preferred for sleep maintenance and the one that is preferred for initiating sleep. Describe the reasoning for these preferences.
|
1. INITIATE SLEEP:
a. Zaleplon (Sonata) for initiating sleep b. Short elimination t ½ vs zolpidem (Ambien) 2. MAINT SLEEP: a. Zolpidem (Ambien) for maint sleep, opp reasons b. Eszopiclone (Lunesta) 1) Long t ½ |
|
|
32. Describe how the pharmacokinetics for “Z drugs” differ from the benzodiazepines.
|
Bind to GABA-A but the DOA not impacted by the formation of long-lived active metabolites
|
|
|
33. Describe how the mechanism of action for ramelteon differs from the “Z drugs”, benzodiazepines, and barbiturates.
|
1. Binds MELATONIN receptor vs GABA-A
|
|
|
34. Describe how piperidinediones are similar to and different from the barbiturates.
|
1. SIMILAR to BARBS
a. Tolerance and dependence b. Induction of δ ALA enzyme involved w/heme biosynth 2. Only advantage over barbiturates is that they have a lower tendency to cause respiratory depression. |
|
|
35. Describe the chemical processes involved in the activation of chloral hydrate to trichloroethanol. (be sure to draw the structure and rxn here)
|
Chloral Hydrate is REDUCED TO TRICHLOROETHANOL (think bis-alcohol to R-OH w/3 Cls)
2. Chloral Hydrate –(-OH)H2O + Cl3C-HC=O –(NAD/NADH)Cl3-CH2OH |
|
|
36. Describe the mechanism that explains how ethanol acts synergistically with chloral hydrate to generate hypnotic effects.
|
1. The oxidation of ethanol to an ALDEHYDE generates NADH, which is necessary for the reduction of trichloroethanal (aldehyde w/chlorinated methyl) to trichloroethanol.
2. “Mickey Finn” is a combination of chloral hydrate and ethanol. |
|
|
examine and consider these GABA medchem structs, identify as many as possible based on SARs
|
none
|
|
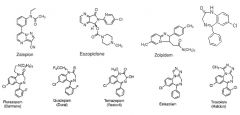
examine and consider these GABA medchem structs, identify as many as possible based on SARs
|
none
|

