![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
1st law thermodynamics |
∆U = q(in) + w(on) |
Equation ∆U=.... |
|
|
Work on |
W(on) = -p X ∆V |
|
|
|
Internal energy with 0 pressure |
∆U = q(p=0) |
|
|
|
Enthalpy= |
H = U + pV |
|
|
|
Internal energy at constant volume |
∆U = q(v) |
|
|
|
Change in enthalpy at constant pressure |
∆H = q(p) |
|
|
|
Ideal gas equation |
pV = nRT |
|
|
|
Work on reversible expansion |
W(on) = -nRT ln[v(f)/v(i)]
Only when pressure changed in very small increments is it reversible |
|
|
|
Work on irreversible expansion |
w(on) = -p X ∆V |
|
|
|
Entropy |
∆S = q(rev)/T
∆S=nR ln[v(f)/v(i)] Measure of state of disorder |
|
|
|
Heat capacity |
Heat energy needed to raise the temperature of an object by 1° C= q/∆T |
|
|
|
Gibbs energy equation |
∆G = ∆H - T∆S |
|
|
|
Open system |
Exchanges energy and matter with surroundings |
|
|
|
Closed system |
Exchanges energy but not matter with surroundings |
|
|
|
Isolated system |
Doesn't exchange energy or matter with surroundings |
|
|
|
Diathermic |
Allows heat to be transferred in/out of system |
|
|
|
Adiabatic |
Doesn't allow heat flow in/out of system |
|
|
|
State functions |
Describe state of system but not how it came to be in that state. -mass, m -Gibbs E, G -volume, V. -Internal E, U -amount, n. -Entropy, S -pressure, p. -temperature, T -enthalpy, H |
|
|
|
Path Functions |
Describe how system transitions between thermodynamic states. Heat energy, q - random molecular motion Work done, w - concerted mm |
|
|
|
0th law thermodynamics |
When 2 objects are independently in equilibrium with a third object, they must also be in equilibrium with eachother. |
|
|
|
Law of E conservation |
1st law thermodynamics: Energy can not be created or destroyed, only transferred from one state to another. ∆U=q(in) +w(on) |
|
|
|
Isobaric |
Constant pressure |
|
|
|
Isochoric |
Constant volume |
|
|
|
Isothermic |
Constant temperature |
|
|
|
Specific heat capacity |
Cs = C/m ( based on mass) |
|
|
|
Molar heat capacity |
Cn=C/n (based on moles) |
|
|
|
Kirchoff's Law |

Finds standard enthalpy change of reaction at different temperatures |
|
|
|
2nd Law of thermodynamics |
The entropy of an isolated system always increases. ∆S = q(rev) / T |
|
|
|
Van't Hoff equation |

Chatelier's principle - system responds to changes |
|
|
|
Third law of thermodynamics |
Entropy of a perfect crystal approaches 0 as absolute temperature approaches 0. |
|
|
|
Entropy of fusion: |
Entropy when solid melts to liquid.
∆S= ∆(fus)H(Tfus) / T(fus) |
|
|
|
Entropy of vaporisation |
Entropy when liquid evaporates to gas
∆S= ∆(vap)H(Tvap) / T(vap) |
|
|
|
1st order rate equation & graph |

|
|
|
|
2nd order rate equation & graph |

|
|
|
|
0th Order rate equation & graph |

|
|
|
|
Half life |
Time taken for concentration of species to half in its initial value |
|
|
|
Half lives for 1st, 2nd, 3rd orders |

|
|
|
|
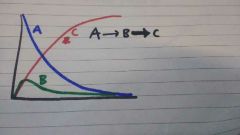
Graph of reactants in steady state |
|
|
|
|
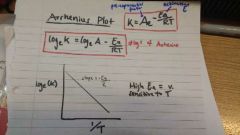
Arrhenius |
|
|
|
|
Schrödinger equation |
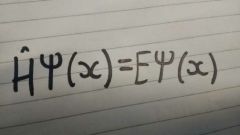
|
|

