![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
|
Isothermal |
Constant temperature (curve on PV - isotherm) |
|
|
Isobaric |
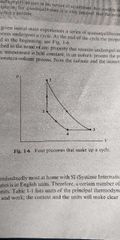
Constant pressure (3->4) |
|
|
Isometric |
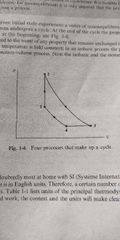
Constant volume (1->2) |
|
|
Pressure (with density) |
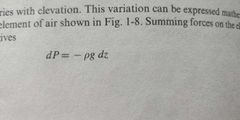
Density * g* hight |
|
|
Gage pressure |
Gage = absolute pressure - atmospheric |
|
|
Value of atmospheric pressure |
101 kPa |
|
|
Absolute zero Kelvin in celcius |
-273.15 |
|
|
Which phase has the highest specific volume |
Vapour |
|
|
Define critical point |

Tip of PV curve after which there's no distinction between liquid and vapour |
|
|
Mass * Volume in terms of specific vapour and liquid volumes |
mv = mf vf + mgvg |
|
|
Dryness fraction x |
x = mg / m v = vf + x(vg - vf) |

