![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
63 Cards in this Set
- Front
- Back
|
What are the 6 organ systems affected by adipocyte-derved factors?
|
1. vasculature
2. muscle 3. brain 4. repro 5. beta cells 6. liver |
|
|
What are the stages of adipogenesis? 4
|
1. Mesenchymal cells ungergo proliferation and determination becoming
2. Preadipocytes that undergo proliferation and differentiation into 3. Immature multiocular adipocytes that hypertrophy into 4. mature unilocular adipocytes |
|
|
From a mesenchymal stem cell what are the 3 options of what they could turn into? and what 3 things determine that?
|
1. osteocyte, adipocyte, and myocyte
2. gene expression, timing, and balance |
|
|
What is the receptor that activates mesenchymal stem cells into adipocytes?
|
Perozone Proliferator Activated receptor. PPAR
|
|
|
What are the 2 phases that adipogenesis is divided into?
|
1. determination - involves the commitment of a pluripotent stem cell to the adipocyte lineage
2. as terminal differentiation, the preadipocyte takes on the characteristics of the mature adipocyte |
|
|
What does the determination phase of adipogenesis result in?
|
This results in the conversion of the stem cell to a preadipocyte, which cannot be distinguished morphologically from its precursor cell but has lost the potential to differentiate into other cell types
|
|
|
What are the characteristics of a mature adipocyte?
|
1. lipid transport and synthesis
2. insulin sensitivity 3. the secretion of adipocyte-specific proteins |
|
|
What are adipokines?
|
- proteins produced by adipose tissue that have autocrine, paracrine, and /or endocrine effects
- some are unique to adipose tissue and others are not |
|
|
What are the adipokines of the adipose tissue involved in the immune system? 3
|
TNF alpha, IL-6, C-reactive protein
|
|
|
What are the adipokines of the adipose tissue involved in growth factors? 2
|
TGF - beta and IGF - 1
|
|
|
What are the adipokines of the adipose tissue involved in the complement pathway? 1
|
adipsin
|
|
|
What are the adipokines of the adipose tissue involved in the appetite and metabolism? 3
|
1. leptin
2. adiponectin 3. resistin |
|
|
What are the adipokines of the adipose tissue involved in the vascular system? 3
|
1. VEGF
2. RAS 3. PAI -1 |
|
|
What are the adipokines of the adipose tissue involved with steroids? 2
|
1. glucocorticoids
2. estradiols |
|
|
What is Leptin? What determines how much is secreted?
|
1. a polypeptide hormone similar to cytokines with long and short receptors that reside in the CNS and peripheral tissues
2. It is secreted in direct proportion to the amount of adipose tissue |
|
|
When is Leptin secretion increased? 4
|
1. insulin
2. glucocorticoids 3. TNF-alpha 4. estrogen |
|
|
When is Leptin secretion decreased? 4
|
1. Beta3-adrenergic
2. androgens 3. Free Fatty acids 4. Growth hormone |
|
|
What is the effect of Leptin?
|
energy intake and expenditure -> satiety factor.
|
|
|
What mediates the effect of Leptin?
|
Hypothalamic pathways
- increased by POMC - decreased by NPY |
|
|
What happens to Leptin in obese pts?
|
elevated in obesity and develops resistance, dec the signaling and is no longer effective as a satiety factor
|
|
|
What peripheral tissues does Leptin effect? 4
|
1. ovary, prostate, testis, placenta
2. immune factors 3. hematopoiesis 4. bone development |
|
|
What increases Leptin satiety factor? 3
|
1. POMC
2. Glucose uptake by skeletal muscle 3. TRH expression and secretion from hypothalamus: important for secretion in puberty |
|
|
What dec the effect of Leptin in satiety factor? 2
|
1. NPY neurons
2. insulin secretion and synthesis of pre-proinsulin |
|
|
What kind of relationship does adiponectin have with insulin resistance and inflammatory states?
|
It has an inverse relationship. The more adiponectin there s the less insulin resistance and inflammatory states there will be.
|
|
|
What are the effects of Adiponectin in the liver? 3
|
1. inc insulin sensitivity
2. dec hepatic glucose output 3. inc Fatty acid oxidation |
|
|
What are the effects of adiponectin in the muscles? 2
|
1. inc. glucose use
2. inc fatty acid oxidation |
|
|
What are the uses of adiponectin in the vascular wall? 4
|
1. dec adhesion molecule expression
2. dec monocyte adhesion 3. dec macrophage transformation into foam cells 4. dec VSMC proliferation |
|
|
What are the 3 effects of Resistin?
|
1. resistance to insulin
2. dec glucose uptake by the liver 3. proinflammatory |
|
|
How is IL-6 implicated in the pathogenesis of obesity and insulin resistance?
|
1. dec secretion of adiponectin
2. dec expression of insulin receptor signaling |
|
|
How does skeletal muscle IL-6 differ from adipose IL-6?
|
In the skeletal m. IL-6 increases glucose uptake and dec inflammation. In adipose and hepatic, IL-6 s proinflammatory and impairs insulin induced IR and IRS1 phorylation thus inhibiting insulin.
|
|
|
What does Caspase-1 do?
|
activates IL-1beta and IL-18 causing the inflammatory state associated with obesty
|
|
|
What is TNFalpha implicated with? 4
|
1. pathogenesis of obesity and insulin resistance
2. inc in obesity 3. dec uptake and storage of NEFA and glucose 4. dec insulin signaling via degradation of IRS 1 and 2 |
|
|
What does PAI-1 inhibit?
|
uPA and tPA
|
|
|
What is PAI-1 (Plasminogen Activator Inhibitor -1 correlated with?
|
1. inc in obesity and insulin resistance may lead to DM II and CVD
2. may promote neointimal growth by dec fibrinolysis thereby stabilizing fibrin matrix for VSMS migration and promting VSMC proliferation |
|
|
How are adipose tissue marcophages (ATMs) connected with CVD and DM?
|
In obese ppl, they lose the ability to store triglycerides well and allow them into circulation. Then, FFAs trigger ATMs to produce proinflamatory cytokines, such as TNF-alpha and PAI-1. These can lead to insulin resistance, which sustains FFA release and feed-forward mechanisms. PAI- causes ectopic fats deposits that inc the risk for thrombi.
|
|
|
What are the changes in adipose tissue, liver, and muscle with obesity and insulin resistance?
|
1. Lean subjects = few macrophages, high adiponectin, and low inflammatory cytokines. β-Oxidation of lipids in muscle is high, and little ectopic fat in the muscle and liver.
2. Obesity and insulin resistance = many macrophages, high adipokines, and low levels of adiponectin. This adipose tissue may be limited in its lipid storage capacity, and this feature, along with the pro-inflammatory state, promotes ectopic lipid accumulation |
|
|
What is the link between the FFA and the Toll Like Receptor 4 pathway?
|
FetA, FFAs and LPS can both stimulate Tlr4 signaling; LPS directly binds the Tlr4-CD14 complex, whereas FFAs bind FetA, which then binds Tlr4. TLR4 signaling leads to the activation of the transcription factors nuclear factor-κB (NF-κB) and AP-1, which can then upregulate the transcription of inflammatory genes, resulting in the production of inflammatory cytokines that can lead to insulin resistance.
|
|
|
Which is worse: viceral or subcutaneous fat?
|
increased visceral adipose tissue is associated with an increased risk of insulin resistance and cardiovascular disease, whereas increased subcutaneous adipose tissue is not
|
|
|
What is the role of 11betaHSD1?
|
the role of 11betaHSD1 is to convert inactive cortisone into active cortisol and glucocorticoids (+) adipocyte formation
|
|
|
What are the main things visceral adipose produces? 7
|
1. IL-6
2. PAI-1 3. Resistin 4. Angiotensinogen 5. ACE 6. 11betaHSD1 7. capase-1 |
|
|
What are the main things subcutaneous adipose produces? 3
|
1. leptin
2. TNF-alpha 3. adiponectin |
|
|
What suppresses appetite? 7
|
1. POMC - proopiomelanocortin
2. CART - cocaine and amphetamine regulated transcript 2. Leptin 3. Insulin 4. PYY -peptide YY 5. GLP1 - glucagon‐like peptide 1 6. Oxm - oxyntomodulin 7. PP - pancreatic polypeptide |
|
|
What increase appetite?
|
1. NPY - neuropeptide Y
2. AgRP - agouti related peptide 3. Ghrelin 4. MCH - , melanin concentrating hormone; 5. Orexins |
|
|
Draw the appetite control schematic.
|
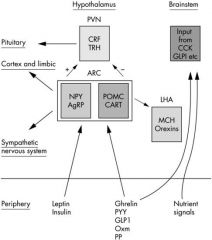
|
|
|
What provides the stimulation of feeding?
|
Neuropeptide Y pathway
|
|
|
What peptide elicits satiety?
|
Cholecystokinin (CCK) released from I cells in the duodenal mucosa in repsonse to fat and protein digestion. It inhibits gastric empying resulting in distention and contributes to the satiety response.
|
|
|
What is the source? site of action? and clinical effects of insulin?
|
1. beta cells
2. Hypothalamus 3. dec appetite, inc metabolism |
|
|
What is the source? site of action? and clinical effects of leptin?
|
1. adipocytes
2. hypothalams 3. dec app, inc metabolism |
|
|
What is the source? site of action? and clinical effects of CCK (Cholecystokinin) ?
|
1. I cells of the duodenum
2. vagal afferents 3. dec appeitie, dec gastric emptying |
|
|
What is the source? site of action? and clinical effects of PYY?
|
1. L cells of the ileum, cecum, colon
2. hypo and vagus 3. dec appetite, inc meta, dec gastric emptying |
|
|
What is the source? site of action? and clinical effects of ghrelin?
|
1. endocrine cells of gastric fundus
2. hypo 3. inc appetite, dec metabolism |
|
|
What is the source? site of action? and clinical effects of PP?
|
1. pancreatic islet PP cells
2. Hypo 3. dec appetite |
|
|
What is the source? site of action? and clinical effects of amylin?
|
1. pancreatic islet PP cells
2. hypo 3. dec gastric empying, dec glucagon, dec appetite |
|
|
What is the source? site of action? and clinical effects of GLP-1?
|
1. L cells
2. Brainstem and Pancreas 3. inc insulin release, dec glucagon, dec gastric emptying, dec appetite |
|
|
What is the source and clinical effects of GIP?
|
1. K cells
2. inc insulin release and dec glucagon |
|
|
What is the source? site of action? and clinical effects of Glucagon?
|
1. pancreatic alpha cells
2. liver 3. inc hepatic glycogenolysis and gluconeogenesis |
|
|
What is the source and clinical effects of oxyntomodulin?
|
1. L cells
2. dec appetite and dec gastric emptying |
|
|
What is metabolic syndrome or syndrome X? 5
|
Complex interrelated rsk factors for developing CVD and DM.
1. High BP 2. Elevated blood glucose 3. high triglycerides 4. low levels of HDL 5. central adipocity |
|
|
What are the clinical criteria for metabolic syndrome? 5
|
waist circumference: ♂ >102 cm (40 in); ♀ >88 cm (35 in)
triglycerides > 150 mg/dL HDL cholesterol <40 mg/dL ♂; <50 mg/dL ♀ blood pressure > 130 / 85 mmHg fasting glucose >100 mg/dl |
|
|
tx for obesity, metabolic syndrome, or type 2 DM?
|
1. lifestyle changes
2. pharm 3. surgery -> baratric |
|
|
What are the pharm interventions for obesity, DM, and metabolic syndrome? 3
|
1. Orlistat (Xenical) - dec gastric and pancreatic lipases, ↓ absorption of undigested triglycerides
2. Lorcaserin (Belviq) - selective agonist of 5-HT2C receptor (satiety factor that can affect metabolism) 3. Phentermine + extended release topiramate (Qsymia) - reduce appetite and may increase metabolism (can cause birth defects: restricted use in US) |
|
|
What are some targets for antiobesity drugs? 3
|
1. leptin
2. cholecystokinin 3. glucagon-like peptide = dec glucagon secretion and inc pancreatic beta cells 4. dipeptidyl peptidase 4 inhibitors - catabolzes GLP-1 5. peptide YY - anorexigenic and dec meal size 6. amylin - limits meal size 7. ghrelin - dec insulin secretion |
|
|
Mechanism behind success of surgery? 3
|
1. Increased secretion of peptide YY and amylin
Amylin can synergize with leptin and peptide YY to increase satiety thereby ↓ food intake 2. Removal of stomach – less ghrelin which is orexigenic primarily secreted from fundus 3. Interposition of ileum – early exposure of ingested food → GLP-1 secretion from L cells: slows gastric emptying (-) apoptosis of pancreatic β cells (+) growth and differentiation of pancreatic β cells plays a role in satiety |

