![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
77 Cards in this Set
- Front
- Back
|
Describe the Hypothalamic-Pituitary-Gonadal axis.
|
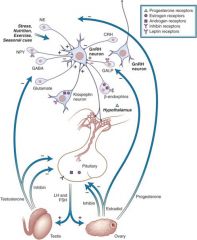
- GnRH from the nasal placode migrates to the hypothalamus and into the anterior pituitary.
- the anterior pituitary releases LH and FSH which acts upon the testis and ovary. - the testis produce inhibin (which inhibits release from the anterior pituitary) and testerone (which inhibits GABA) - the ovary produces inhibin (inhibits ant. pit.), Estradiol (inhibits anterior pit. and GALP), and progesterone (which inhibits as well) |
|
|
What stimulates the release of LH and FSH?
|
GnRH or LHRH (luteinizing hormone releasing hormone), released in pulsatile pattern and originate outside of the CNS
|
|
|
How does the pulsatility of GnRH effect Gonadotropin secretion?
|
LH - is released rapidly
FSH - is released more slowly |
|
|
What hormones are associated with what stage?
|
Estradiol = follicular stage
Progesterone = luteal phase |
|
|
What is the menstrual cycle?
|
- a 28 (21-35) day cycle divided between a follicular phase and a luteal phase. The follicular phase marks the beginning of the cycle, and starts during menses.
- (While the length of the follicular phase can vary, the luteal phase always precedes the onset of menses by 14 days) |
|
|
What are the histological stages of the follicular phase? 4
|
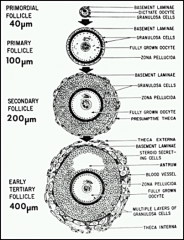
Primordial follicle → Primary follicle → Secondary follicle → Graafian follicle, with atresia of neighboring follicles.
|
|
|
Describe folliculogenesis: the primordial follicle.
|
-Primordial follicle - oocyte development has been arrested in meiosis I (prophase I).
- It is surrounded by squamous cells. As the follicle is stimulated to grow, the oocyte becomes larger and the flat squamous cells begin to become cuboidal. |
|
|
Describe folliculogenesis: the primary follicle.
|
- the flat squamous cells have became cuboidal cells that have differentiated into granulosa cells.
- the granulosa cells begin to grow and divide to eventually create a basement mem. - the zona pellucida forms - oocyte is fully grown STAGE DETERMINED WHEN GRANULOSA CELLS SURROUND THE OOCYTE. |
|
|
Describe folliculogenesis: the secondary follicle.
|
- the basement laminae grows and separates the stromal layer and the developing thecal layer
- granulosa grows surrounding the oocyte and divides it from the thecal layer |
|
|
Describe folliculogenesis: the tertiary follicle/graafian. (antral).
|
- the theca externa develop and secrete hormones
- antrums develop - fluid filled cavities w/ waste and nutrients b/c the thecal cells are vascular but the rest of the follicle is not. |
|
|
What is a Graafian cell?
|
A large antral follicle
|
|
|
What is the timeline for follicular development?
1. Primordial to primary? 2. Primary to secondary? 3. Secondary to early antral? 4. Early Antral to Graafian? |
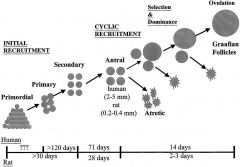
1. Primordial to primary = 150 days
2. Primary to secondary = 120 days 3. Secondary through early antral = 71 days 4. Small Antral follicles to Graafian = 14 days If women are on replacement hormones and stop them, they might still have an effect for months afterwards. |
|
|
What are the 2 gonadotropins and 2 cells involved follicular steroidogenesis?
|
2 cell: 1) theca
2) granulosa 2 gonadotropins: 1) LH 2) FSH |
|
|
What cell produces Estradiol? And which receptor does it have? What is the other cell?
|
1. Granulosa produces Estradiol and has the FSH receptor
2. Theca cells produce Androstenedione and have a LH receptor |
|
|
What is the relationship between the Theca and granulosa cell?
|
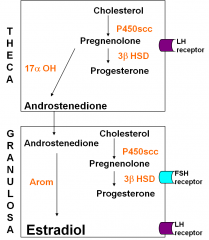
Granulosa cell is dependent on the theca cell for androgen b/c it doesn’t have 17 alpha OH. Theca don’t have aromatase so they cant produce estrogen, theca only produce androgens. Both cell types are needed.
Granulosa develop LH receptors and can pump out even more estradiol. |
|
|
What is Inhibins? Action?
|
- peptide hormones produced by granulosa cells and luteal cells
- inhibits FSH |
|
|
What the hell is AMH? Action? Why do we measure it?
|
1. produced by granulosa cells from 2º stage to small antral stage of development
2. restraining influence on growth of primordial / primary follicles 3. used to assess follicular reserve; hyper-/hyposecretory states |
|
|
Explain the roles of hormones during the follicular stage.
|
1. Follicles will begin to grow and inc their numbers. They then produce estradiol and inhibin.
2. These feedback at hypothalamus and dec LH and FSH. 3. Even though they might be in the same stage, follicles are at different times in that stage. Some may "die" when FSH is dec but others may keep going. 4. Even higher levels of estradiol, select out for only 1 cell that has reached the pt in development w/ LH and FSH receptors. This final cell continues to produce Estradiol to inhibit other antral cells. 5. At this stage, a final surge of LH will release the oocyte, triggers luteinization of both granulosa and theca cells. |
|
|
What ruptures a follicle, releases an oocyte into ovulation, and causes lutinization?
|
LH surge.
|
|
|
What occurs w/ oocyte maturation? 2
|
resumption of meiosis
cytoplasmic maturation |
|
|
What occurs w/ luteinization? 4
|
increased StAR
increased P450scc, 3βHSD increase in LH receptors increase ER and mitochondria |
|
|
What occurs w/ ovulation? 2
|
1. progesterone receptor - COX-2 (PGF2α and PGE2)
2. proteolytic enzymes - plasminogen activator |
|
|
What is the MOA behind the ovulatory process? The role of FSH?
|
FSH stimulates Cumulus expansion.
|
|
|
What is the MOA behind the ovulatory process? The role of LH?
|
1. LH stimulates:
- resumption of meiosis - progesterone receptor =>stimulates COX-2 receptor =>prostaglandin => inflammation/lysosomal enzymes => presumptive stigma - luteinization => stimulates Granulosa lutein cells and theca lutein cells THIS ALLOWS FOR THE OOCYTE TO RUPTURE FROM THE FOLLICLE FROM THE STIGMA. |
|
|
When is meiosis fully completed?
|
arrested in meiosis I then continues to grow until meiosis II then completes development when fertilized
|
|
|
What is the cumulus expansion? Why is it important?
|
The granulosa cells after the LH surge begin to spread out, allowing for sperm to penetrate the egg.
|
|
|
After the developing egg is ovulated, what happens to the remaining follicle?
|
it regresses to form a corpus luteum which synthesizes and secretes progesterone that causes proliferation of arteries and glandular secretions from the endometrial lining (helps prepare the endometrium for receipt of a fertilized egg).
|
|
|
When does ovulation occur?
|
regardless of length of cycle, always 14 days before menses
|
|
|
What happens if fertilization does not occur?
|
If fertilization does not occur, the corpus luteum regresses at the end of the luteal phase, and estradiol and progesterone levels decrease abruptly. This sudden decrease in hormones causes shedding of the endometrial lining and the onset of menses.
|
|
|
What does the corpus luteum produce?
|
progesterone and lots of it
|
|
|
Might want to look at Komar's nifty lil drawing...
|
I think it helps somewhat.
|
|
|
I love pictures...let's put it alllll together now.
|
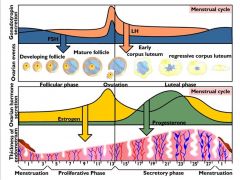
|
|
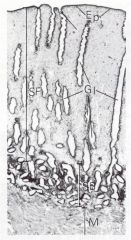
Histo for the hell of it...
Ep=epithelium Gl=glands SF=stratum functionale SB=stratum basale M=myometrium |
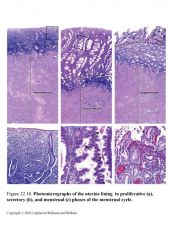
and why not a lil more
|
|
|
When does full boobie development occur?
|
preggo and lactation
|
|
|
What role does estradiol play in the development of breast tissue? 2
|
proliferation of epithelium; developmet of ducts
|
|
|
What role does progesterone play in the development of breast tissue? 3
|
branching of ducts and proliferation of alveoli; synergizes w/ estradiol to maximize epithelial development
|
|
|
What role does oxytocin play in the development of breast tissue?
|
promotes milk secretion by (+) contraction of smooth muscle cells to move milk from glands into ducts
|
|
|
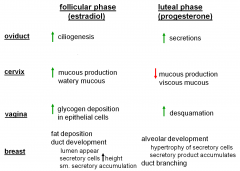
A review chart...
|
|
|
|
What effects female repro system? 4
|
Age
Menopause Polycystic Ovary Syndrome (PCOS) Obesity |
|
|
How does age effect female fertility? 3
|
1. Late 30s: effects of age on fertility become significant
2. Pool of primordial follicles decreases - Reduced inhibin production, eventually, reduced estradiol production - Decrease in (-) feedback to hypothalamus/pituitary ( Increased FSH nd LH) Oocyte is a 1° factor contributing to sub-/infertility as females age => Meiotic nondisjunction - Reduced formation of chiasmata during fetal oogenesis - Accumulation of reactive oxygen damage during prolonged interval until ovulation |
|
|
What are the consequences of vitro fertilization?
|
maternal Ovarian hyperstimulation syndrome
affects up to 10% of women who go through IVF - cause ? - ↑ permeability of capillaries in ovary which leads to loss of protein rich fluid from intravascular compartment to interstitial space - hCG treatment associated with risk mild: abdominal discomfort; elevated E2 and P4 moderate: abdominal distension; diarrhea; vomiting severe: electrolyte imbalances; ascites; shock; acute respiratory distress syndrome |
|
|
What are the consequences of vitro fertilization? maternal mortality.
|
maternal mortality rates are rising in the US and elsewhere
rationale?: today more pregnant women have health problems – diabetes, obesity, and many are older : the reasons why a woman undergoes IVF may account for the increases risk of death |
|
|
Consequences of ART: offspring. 6
|
1. increased risk of Angelman syndrome: a genetic disorder characterized by severe mental retardation, seizures, ataxic gait, jerky movements, lack of speech, microencephaly, and frequent smiling and laughter
2. increased risk of Beckwith-Wiedemann syndrome: a congenital (present from birth) growth disorder that causes large body size, large organs, and other problems. 3. increased risk of cerebral palsy 4. higher systolic and diastolic bp, and fasting glucose compared with age matched naturally conceived controls 5. altered body fat composition in children conceived by IVF compared with age matched naturally conceived controls 6. altered pattern of DNA methylation between children conceived in vitro versus in vivo |
|
|
Menopause reflects changes where?
|
reflects changes at the level of the ovary AND hypothalamus / pituitary
|
|
|
What is Menopause?
|
1. decrease in number of ovarian follicles below threshold necessary to maintain ovulatory cycles
2. changes in the neuroendocrine axis that are independent of altered ovarian steroidogenesis - age related changes in responsiveness to GnRH - hypothesized change(s) in effects of E2 (+) feedback |
|
|
What is Polycystic Ovary Syndrome (PCOS)? sx? 3 biochem features? 5 KNOW.
|
1. obesity, hirsutism, and amenorrhea frequently associated
2. Biochemical features (compared to normal early follicular phase) ↑ LH ↓ or ↔ FSH ↑ androgens ↔ inhibins ↑ or ↔ estradiol |
|
|
What is the number 1 cause of infertility n premenopausal women? KNOW.
|
Polycystic Ovary Syndrome (PCOS)
|
|
|
NIH criteria for diagnosis: Polycystic Ovary Syndrome?
|
1) clinical evidence of hyperandrogenism and/or hyperandrogenemia
- hirsutism; acne; alopecia… 2) oligoovulation 3) exclusion of other disorders - hyperprolactinemia; thryoid dysfunction; CAH; tumor… |
|
|
Rotterdam criteria for diagnosis (2003): 2 of the following 3: Polycystic Ovary Syndrome?
|
1) oligo- or anovulation
2) clinical and/or biochemical evidence of hyperandrogenism 3) polycystic ovaries |
|
|
What is the principle abnormality of polycystic ovary syndrome?
|
- (may also involve adrenal androgen production but minor)
- hyperandrogenemia - may facilitate visceral fat deposition - visceral fat ↑ lipolytic activity - - - ↑ FFA - increase in FFA may lead to insulin resistance in muscle - (-) SHBG syn. - - - more androgens free in circulation |
|
|
Polycystic Ovary Syndrome: abnormal insulin resistance.
|
- hyperinsulinemia ? due to post-receptor signaling lesion - ↑ ovarian androgen production
- ↑ basal cell growth of skin (acanthosis nigricans) - affects on vascular and endothelial cells (hypertension…) - affects liver and peripheral lipid metab (dyslipidemia…) : (-) SHBG production by liver |
|
|
What are the insulin signaling defects in polycystic ovary syndrome?
|
- The signaling defect is due to serine phosphorylation of the insulin receptor and IRS-1 secondary to intracellular serine kinases. This results in decreased insulin-mediated activation of PI3-K and resistance to the metabolic actions of insulin. There is constitutive activation of kinases in the ERK/MAPK mitogenic pathway in PCOS, and these kinases contribute to inhibitory serine phosphorylation of IRS-1 in PCOS skeletal muscle. Serine phosphorylation of P450c17 increases its activity, and it has been postulated that the same kinase may inhibit insulin signaling and increase androgen production in PCOS.
|
|
|
What is Hyperthecosis in polycystic ovary syndrome?
|
- inc thecal androgen production
↑ LH (+) theca cell androgen production Insulin (+) theca cell androgen production Elevated androgens enhance number of small, healthy follicles |
|
|
Do Androgens ↑ sensitivity to GnRH ?
|
Anovulation due to too many small, antral follicles changing endocrine environment - - inhibin and estradiol keep FSH in check
Anovulation - - No CL - - No progesterone - - lack of (-) on GnRH secretion |
|
|
What are the clinical problems associated with PCOS? 3
|
Women with PCOS at increased risk for
: hyperovarian stimulation syndrome : developing gestational diabetes : developing preeclampsia |
|
|
What is the proposed pathophysiology of PCOS?
|
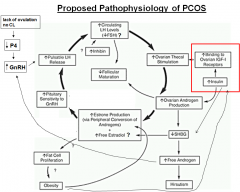
|
|
|
Prepregnancy maternal obesity increases risk of: 3.
|
- fetal growth abnormalities
- stillbirth (by 2-5 fold) - Risk of having a child born with congential heart defect(s) increases dramatically with BMI in obese women (BMI ≥ 30 kg/m2) |
|
|
Obese women risk factors 4
|
Reduction in fertility
lower success rate with ART increase maternal mortality greater risk of miscarriage |
|
|
Can maternal under- OR over-nutrition can predispose offspring to develop metabolic syndrome (obesity; type II diabetes)?
|
Yes, maternal under-nutrition during pregnancy can lead to a premature leptin surge and a lack of leptin.
- overnutrition can lead to maternal hyperleptinemia and leptin resistance. |
|
|
Where do GnRH neurons originate? What problem can this lead to?
|
GnRH neurons originate OUTSIDE of CNS
- originate from nasal placode - migrate into hypothalamus during development Kallman syndrome occurs (hypogonadism) when this development is improper...pt will not be able to smell either |
|
|
as follicles grow in size in the female, what do they release? what is the effect of this? how does it change? what does this lead to?
|
Estradiol---inhibitory of GnRH
when it reaches a critical threshold it becomes stimulatory of the GnRH this leads to LH/FSH surge causing ovulation and formation of corpus luteum |
|
|
Theca cells have what type of receptor? What does this lead to the production of? where is this brought to?
|
LH receptor
produces Androstenedione brought to granulosa cells |
|
|
Granulosa cells have what receptor? what does this lead to the production of? What else can they produce (and where did they receive the starting product)?
|
FSH receptor
produces progesterone also produces Estradiol from conversion of androgens made in the Theca cells |
|
|
17 alpha OH is in what type of cell? Leads to the production of?
|
Theca
Androgens |
|
|
aromatase is in what type of cell? Leads to the production of?
|
Granulosa
estradiol (GnRH-->FSH-->Granulosa-->aromatase-->estrodiol) |
|
|
starting with cholesterol, go through production of estrogens
|
cholesterol-->progestins-->androgens-->estrogens
|
|
|
as a follicle matures, why is it able to produce large amounts of estradiol?
|
the granulosa cell gains an LH receptor (it already had an FSH receptor)
this helps produce more estradiol (not really sure how...) remember you convert androstenedione to estradiol in these granulosa cells |
|
|
what properties will a follicle have that is able to continue to grow and develop into the dominant follicle in the menstrual cycle?
|
one that has both FSH and LH receptors on the granulosa cell, and can produce large amounts of estrodiol
|
|
|
increased StAR is associated with what process? Leading to?
|
Leteinization
increased progesterone |
|
|
Please run through the menstrual cycle.
|
LH/FSH hits the follicles--> increases estradiol and inhibin
One follicle is able to grow a bit more than the others (more mature) and continues to produce estrodiol and inhibin (maintaintaing an inhibitory affect on LH/FSH) maturing follicle then gains LH/FSH granulosa site leading to an even bigger release of estrodiol causing the threshold to be reached when Estrodiol stops being inhibitory and instead causes the LH/FSH surge ovulation occurs and the corpus luteum produces a whole bunch of Progesterone if the egg is not fertilized you have menses and the process starts over |
|
|
in the oviduct, what happens due to the follicular phase (estradiol) and luteal phase (progesterone)
|
follicular: increase ciliogenesis
luteal: increase secretions |
|
|
in the cervix what happens due to the follicular phase (estradiol) and luteal phase (progesterone)
|
follicular: increase watery mucous production
luteal: decrease viscous mucus |
|
|
in the vagina, what happens due to the follicular phase (estradiol) and luteal phase (progesterone)
|
follicular: increase glycogen deposits in epitheial cells
luteal: increased desquamation |
|
|
in the breast, what happens due to the follicular phase (estradiol) and luteal phase (progesterone)
|
follicular: fat deposition, duct development
luteal: alveolar development, duct branching |
|
|
String of pearls on ovary =
|
Polycystic Ovary Syndrome (PCOS)
|
|
|
an obese woman presents to your office saying that she is having issues getting pregnant. She has notable hirsutism and ultrasound of her ovaries shows string of pearls....what she got? 2 possible mechanisms cause this problem?
****** |
Polycystic Ovary Syndrome (PCOS)
Insulin resistance or Theca cell androgen production |
|
|
what is cumulus expansion? 2 things this allows for?
|
egg has ovulated
cumulus become more diffuse in their surrounding of the egg this allows the fibrae to easily pull the egg into the ovaduct secondly it allows for sperm to enter the egg more easily |

