![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
64 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Democritus of Abdera |

Said all things are made of tiny indivisible units called atoms |
No experimental support-philosopher |
|
|
Dalton |

Solid ball, atomic theory |
|
|
|
True or False? Atomic Theory From Dalton Compounds have 2 or more different kinds of atoms |
True |
|
|
|
True or False? Atomic Theory From Dalton A chemical reaction is the rearrangement of atoms |
True |
|
|
|
True or false? Atomic theory from Dalton All matter is made of atoms that are indivisible & indestructible |
False |
|
|
|
Thompson |

Plum pudding model, cathode tube, discovered the electron |
|
|
|
Millikan |
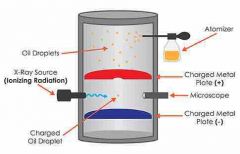
Oil-drop experiment, 1st accurate measure of electrons charge and mass |
|
|
|
Ernest Rutherford |

Dense center, electron shell, shot alpha particles at a piece of gold some bounced back. Idea of the nucleus-core of the atoms with protons and neutrons inside, all the weight very dense, positive, electron orbits are very small |
|
|
|
Most of the atom is? |
Empty space |
|
|
|
Bohr |
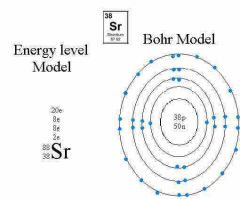
Energy levels, said electrons travel in set circular paths around nucleus Each path needs a different amount of energy. The farthest from the nucleus the electron is the more energy |
|
|
|
Schrodinger |

Today's idea: Quantum Mechanical Model "electron Cloud Theory" Electrons are only found in certain areas called clouds. each cloud is determined by the probability of an electron been there 90% of the time since an electron is so small it's exact position is difficult to know |
|
|
|
Atom |
Smallest basic unit of matter |
|
|
|
Element |
Made up of compounds |
|
|
|
Compound |
Made up of elements two or more elements combined |
|
|
|
Solid |
Definite shape and definite volume not easily compressed Atoms packed together, most dense |
|
|
|
Liquid |
No definite shape, free-flowing but close items, can diffuse. Does it compress but flows |
|
|
|
Gas |
No definite shape, always fills space, Atoms are the most separated. The least or no attractive force |
|
|
|
Mixtures |
Can be separated, not permanent, 2 things that are together but not permanently |
|
|
|
Heterogeneous (vinegar, and sand together) |
A mixture of two or more compounds. The mixture is not uniform. |
|
|
|
Heterogeneous (vinegar, and sand together) |
A mixture of two or more compounds. The mixture is not uniform. |
|
|
|
Suspension |
All components are in the same phase |
|
|
|
Homogenous (koi laid + water and air) |
Settles together. Completely uniform. |
|
|
|
Heterogenous separation by (3 things) |
Filter, magnet, centrifugation |
|
|
|
Homogenous separation by (1 thing) |
Distillation |
|
|
|
Chemical property (paper can burn, gas burning. |
The way a substance will react with others to form a new compound with different properties -smell -burning -smoke -changing original/ making something and adding something
|
|
|
|
Physical property (Ice is a solid, paper is a solid. Paper has a density.) |
A characteristic that can be observed and measured without changing its composition. Hardness, strength, conductivity |
|
|
|
Water boiling= |
Physical change |
Water is still water even after burning. |
|
|
Scientific methods (6 steps) |
1. problem 2. Hypothesis 3. Experiment 4.Analyze 5. Accept or reject the hypothesis 6. Communicate |
|
|
|
Theories (Big Bang theory, theory of evolution) |
Long explanations that are proven |
|
|
|
Laws (Laws of Gravity) |
Short equation (if wrong) |
|
|
|
**ADDING and SUBTRACTING sig fig rules*** |

*Keep same place value *keep scientific notation |
|
|
|
Rules for MULTIPLYING AND DIVIDING SIG FIGS |
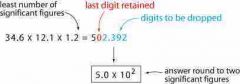
|
|
|
|
Quantitative |
(Measurement) measure liquid with cylinder |
|
|
|
My King Henry Died By Drinking Chocolate Milk Mirco |
Mega, kilo, hecto, deca, base (m,L, g), deci, centi, mili, micro |
|
|
|
Length= |
Meters (m) |
|
|
|
Volume |
Liters=(L) |
Defined by linear measurement 1L |
|
|
Mass |
Grams=(g) |
|
|
|
Chemical change* |
Will get a permanent change their results in a totally new substance |
|
|
|
Density formula |
D=m/v |
|
|
|
Density can be manipulated by |
Volume and heat |
|
|
|
Hot= |
Less dense |
|
|
|
Cold= When water freezes to ice it gets more dense |
More dense |
|
|
|
Elements are different because of the number of _____ in the nucleus |
Protons |
|
|
|
Number of electrons |
= number of protons and atomic number |
|
|
|
Number of protons |
Atomic number |
|
|
|
Atomic mass |
Total number of Protons + Neutrons |
|
|
|
Number of neurons |
Atomic mass-atomic number |
|
|
|
Ions |
Element has gained or lost electrons |
|
|
|
Physical change* |
Will change the look or size, but not the chemical make up |
|
|
|
Cation |
A postively charged atom (lost electrons) (+1= -1) |
|
|
|
Anion |
A negatively charged atom (gained electrons) (-1=+1) |
|
|
|
Isotopes |
Atoms of the same element with different numbers of NEUTRONS. |
|
|
|
Number of protons |

Atomic number |
|
|
|
Atomic mass (Average of all isotopes)caliche fe |
% abundance x isotope + % abundance x isotope -----------------——————— Atomic mass |
|
|
|
Number of neurons |
Atomic mass-atomic number |

|
|
|
How to calculate frequency and wavelength |
|
|
|
|
Light wave idea |
Christian Hugh's |
|
|
|
**Significant figure rules:** |
*Anything before zero= not significant *Anything that is a not 0=significant *If there is a 0 after a "." And a significant number that 0 is significant *If a zero is sandwiched between two significant numbers=significant |
|
|
|
**ADDING and SUBTRACTING sig fig rules*** |
*Keep same place value *keep scientific notation |
|
|
|
Rules for MULTIPLYING AND DIVIDING SIG FIGS |
|
|
|
|
Atomic mass (Average of all isotopes)caliche fe |
% abundance x isotope + % abundance x isotope -----------------——————— Atomic mass |

|
|
|
How to calculate photon energy |
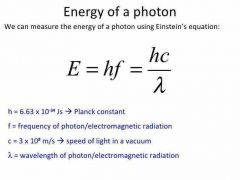
|
|
|
|
How to calculate frequency and wavelength |

|
|
|
|
(Lavoisier's) Law of Conservation: |
Matter cannot be created or destroyed |
|

