![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
What is matter?
How do you classify matter as a pure substance, compound, heterogeneous mixture, homogeneous mixture? |
Matter is anything that has mass and occupies space.
Pure substances have a fixed composition and a unique set of properties. Compounds are pure substances that contain more than one element. Heterogeneous are nonuniform mixtures where composition varies. Homogeneous are uniform where it has the same composition. |
|
|
Separation techniques?
|
Filtration: used to separate a heterogeneous solid-liquid mixture;passed through a barrier w/fine pores (filter paper)
Distillation: separates homogeneous solid-liquid mixture;liquid vaporizes, leaving a residue of the solid in the distilling flask Chromatography: separates all mixtures Gas-Liquid Chromatography: mixture of volatile liquids and gases are introduced into one end of a heated gas tube Decant: pour off the dense liquid |
|
|
Qualitative vs. Quantitative
|
qualitative: description
quantitative: number/amount |
|
|
Precision vs. Accuracy
|
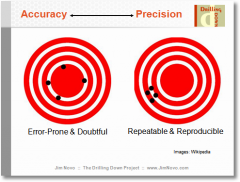
accuracy: closer to true value
precision: reproducibility no matter how true to value |
|
|
Scientific Notation
|
C x 10^n
shorter way of writing large numbers |
|
|
What are SI units, prefixes, and their values?
|
Uses the Metric System
based on increments of 10 length:meter(m) volume: cubic centimeters, liters, milliliters (cc, L, mL) mass:kilograms(Kg) time:second(s) amount: moles(mol) temperature: Kelvin(K) electrical current: ampere(A) luminous intensity:candela(cd) K(kilo)H(hecto)D(deca)b(base)d(deci)c(centi)m(milli)..n(nano)..|u(m, mu) |
|
|
Convert degrees C/F to degrees K
|
C--> 5/9 (F-32)
F-->9/5C+32 K-->C+273.15 (true measure, no degrees for Kelvin) |
|
|
Significant Figures
|
1) non-zero numbers are significant
2)zero's between significant figures are also significant 3)zero's at the end of a number RIGHT of the decimal are significant 4)zero's at the end of a number LEFT of the decimal are NOT significant (place holders) 5)all zeros before the first non-zero number are NOT significant (place holders) 6)count has an infinite number of significant figures or zero significant features (ex. 115 cars) |
|
|
density calculations
|
d=m/v
m=(d)(v) d=(m)(v) |
|
|
conversion factor
|
given x want/given = want
|
|
|
Physical vs. Chemical Changes
Intensive vs. Extensive Changes |
phys:a change in a substance that does not involve a change in identity of a substance
chem:a change in which one or more substances are converted into different substances inten:independent on the amount ex. boiling point exten:dependent on the amount ex. mass |
|
|
Law of Conservation of Matter
|
mass cannot be created not destroyed
|
|
|
Law of Constant Composition (Definite Proportions)
|
regardless of amount, compound has same elements in the same properties by mass
|
|
|
Law of Multiple Compositions
|
Same elements can be in different ratios to create different compounds
ex. carbon dioxide & carbon monoxide |
|
|
John Dalton - 1808 - Atomic Theory
|
1) All matter is composed of atoms
2)Atoms of the same element are identical in size, mass, and other properties; atoms of different elements differ in their properties 3)Atoms cannot be subdivided, created or destroyed 4)Atoms of different elements combine in simple ratios t0 form chemical compounds (H20) 5)In chemical reactions, atoms are combined, separated, and rearranged |
|
|
Modern Atomic Theory
|
The discovery of subatomic particles that make up atoms
The discovery of isotopes |
|
|
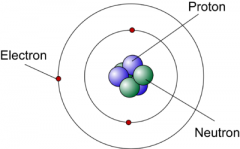
Protons
Electrons Neutrons |
+ | inside the nucleus
- |in the electron cloud (orbitals) no charge | inside nucleus |
|
|
Atomic Number
|
The number of protons in the nucleus of each atom, this never changes
|
|
|
Atomic Mass Number
|
The number of protons and neutrons in the nucleus of the atom
|
|
|
Isotopes (nucleotides)
|
Atoms of the same element that differ in number of neutrons in the nucleus, hence they have different masses
|
|
|
Ions
|
When you lose or gain electrons
Cations: +, loses e- Anions: -, gains e- |
|
|
J.J. Thompson - Late 1800s
|
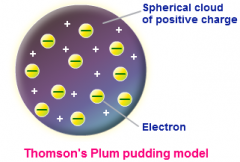
Discovered the electron, used a cathode ray tube to deduce presence of a negatively charged particle.
|
|
|
Earnest Rutherford - Early 1900s
|
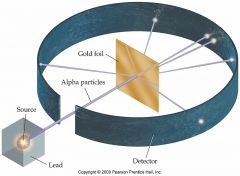
Student of Thompson; found that most particles were passed through, few were slightly deflected, very few were greatly deflected
|
|
|
Rutherford Atomic Model
|
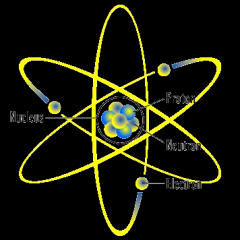
|
|
|
Neils Bohr - Early 1900s
|
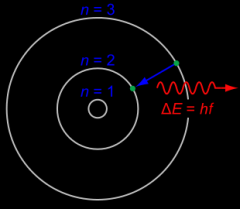
Student of Rutherford; as you get higher in the levels., the areas are closer
|
|
|
Bohr's energy levels
|
|
|
|
Erwin Schrödinger - 1900s
|
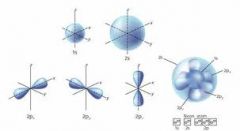
Quantum Mechanical Model
Mathematical model of the atom; an electron cloud is a volume of space where an electron COULD be located |
|
|
Robert Milikan
|
discovered the mass of an electron and charge
mass of e-=1/1840 H atom 1 negative charge |
|
|
James Chadwick - 1932
|
discovered the neutron(has no charge)
|

