![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
Dalton's Model |

Atoms are solid spheres & unique to specific elements |
|
|
Thomson's model |
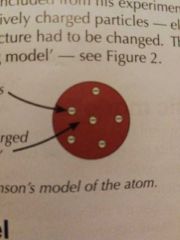
Plum Pudding |
|
|
Rutherford's model |
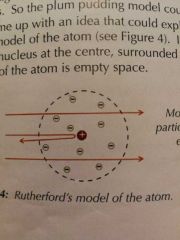
Tiny positive nucleus, cloud of negative electrons. Mostly empty space. Gold foil experiment. |
|
|
Bohr's Model |
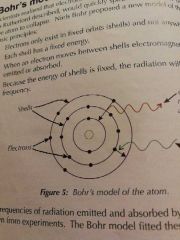
Electrons orbit in fixed orbitals / shells (so they don't collapse) & emit/absorb radiation if they move between shells |
|
|
Define: Relative Atomic Mass (Are) |
Average mass of an atom of an element on a scale where an atom of 12C is exactly 12 |
|
|
Relative Isotopic Mass |
The mass of an atom of an isotope of an element on a scale where an atom of 12C is exactly 12 |
|
|
Relative Molecular Mass (Mr) |
Average mass of a molecule on a scale where an atom of carbon 12 is exactly 12 |
|
|
Relative formula mass |
Average mass of a formula unit on a scale where an atom of 12C is exactly 12. Used for ionic or giant covalent. |
|
|
List Stages: Mass Spectrometry |
Ionisation Acceleration Ion Drift Detection |

