![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
|
Energy flows into an ecosystem as _________ and ultimately leaves as __________ |
Sunlight Heat |
|
|
A partial degredation of sugars or other organic fuel that occurs without the use of oxygen. |
Fermentation |
|
|
The most efficient catabolic pathway? |
Aerobic respiration |
|
|
Oxygen is consumed as a reactant along with the organic fuel? |
Aerobic Respiration |
|
|
The harvesting of chemical energy without oxygen? |
Anaerobic Respiration |
|
|
Cellular respiration technically refers to _________ but is primarily synonymous for ________ |
Aerobic and Anaerobic processes Aerobic Processes |
|
|
Process of aerobic reaction? |
Organic Compounds + Oxygen -> Carbon Dioxide + Energy |
|
|
Degradation of glucose? |
C6H12O6 + 6 O2 -> 6 CO2 + 6 H2O + Energy (ATP & Heat) |
|
|
Is the breakdown of glucose an exergonic or endergonic reaction? |
Exergonic (-686 kcal/mol) |
|
|
How do the catabolic pathways that decompose glucose and other organic fuels yield energy? |
The transfer of electrons during chemical reactions releases energy stored in organic molecules, this energy is ultimately used to synthesize ATP. |
|
|
Oxidation-reduction reactions (redox reactions) |
The transfer of one or more electrons (e-) from one reactant to another. |
|
|
The loss of electrons from one substance in a redox reaction? |
Oxidation |
|
|
The addition of electrons to another substance in a redox reaction? |
Reduction |
|
|
Reducing agent |
The electron donor of a redox reaction |
|
|
Oxidizing agent |
The acceptor of electrons in a redox reaction |
|
|
Redox reaction between Methane and Oxygen Define the (a) Reactants (b) Products (c) Reducing agent (d) Oxidizing Agent (e) Becomes oxdized (f) Becomes Reduced |

|
|
|
Which is oxidized and which is reduced? (a) NAD+ (b) NADH |
(a) oxidized (b) reduced |
|
|
Coenzyme that acts as an electron carrier |
Nicotinamide adenine dinucleotide (NAD+) |
|
|
How does NAD+ trap electrons from glucose and the other organic molecules in food? |
Enzymes called dehydrogenases remove a pair of hydrogen atoms (2 electrons and 2 protons) from the substrate, thereby oxidizing it. The enzyme delivers the 2 electrons along with 1 proton to its coenzyme, NAD+. The other proton is released as a hydrogen ion (H+) into the surrounding solution.
H-C-OH + NAD+ ----Dehydrogenase---> C = O + NADH + H+ |
|
|
The most versatile electron acceptor in cellular respiration |
NAD+ |
|
|
Electron Transport Chain |
Consists of a number of molecules, mostly proteins, built into the inner membrane of the mitochondria of eukaryotic cells. |
|
|
Is electron transfer from NADH to oxygen an exergonic or endergonic reaction? |
Exergonic (free energy change of -53 kcal/mol) |
|
|
During cellular respiration, most electrons travel what route? |
Glucose -> NADH -> Electron Transport Chain-> Oxygen |
|
|
Three stages of cellular respiration? |
Glycolysis Pyruvate Oxidation and the Citric Acid Cycle Oxydative Phosphorylation : electron transport and chemiosmosis |
|
|
Substrate level phosphorylation |
ATP synthesis that occurs when an enzyme transfers a phosphate group from a substrate molecule to ADP, rather than adding an inorganic phosphate to ADP as in oxidative phosphorylation |
|
|
Glycolysis (a) Phases (b) Occurs where? (c) Starting products (d) End products |
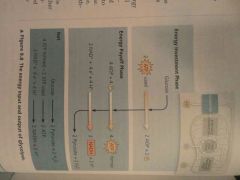
(a) Energy invesment phase, Energy payoff phase (b) Cytosol (c) Glucose, 2 ATP, (2 NAD+) + (4 E-) + (4 H+) (d) 2 pyruvate, 2 H2O, 2 ATP (4 formed - 2 used), 2 NADH + 2 H+ |
|
|
How many steps are involved in the glycolytic pathway? |
10 |
|
|
What is pyruvate converted to upon entering the mitochondrion? |
Acetyl coenzyme A (acetyl CoA) |
|
|
Pyruvate oxidation |
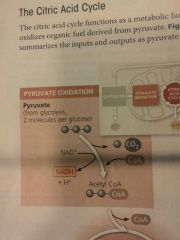
Carried out by a multienzyme complex that catalyzes three reactions: 1. Pyruvate's carboxyl group (--COO-), which is already fully oxidized and thus has little chemical energy, is removed and given off as a molecule of CO2. This is the first step in which CO2 is released during respiration. 2. The remaining two-carbon fragment is oxidized, forming acetate (CH3COO-, which is the ionized form of acetic acid). The extracted electrons are transferred to NAD+, storing energy in the form of NADH. 3. Finally, coenzyme A (CoA), a sulfur-containing compound derived from a B vitamin, is attached via its sulfur atom to the acetate, forming acetyl CoA, which has a high potential energy; in other words, the reaction of acetyl CoA to yield lower-energy products is highly exergonic |
|
|
Prosthetic groups |
Nonprotein components essential for the catalytic functions of certain enzymes. |
|
|
Citric Acid Cycle (a) 2 other names (b) Occurs where? (c) Starting products (d) Ending products |
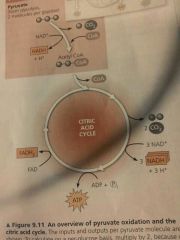
(a) Krebs Cycle, tricarboxylic acid cycle (b) Mitochondrion (c) 2 Pyruvate (d) 4 CO2, 6 NADH, 2 FADH2, 2 ATP (per glucose, half all numbers per pyruvate) |
|
|
What makes the citric acid cycle a cycle? |
The regeneration of oxaloacetate |
|
|
How many steps are involved in the citric acid cycle |
8 |
|
|
What is the only member of the electron transport chain that is not a protein? |
Ubiquinone (coenzyme Q -CoQ) |
|
|
Cytochromes have what prosthetic group? |
heme (contains iron) |
|
|
What electron transporters (and prosthetic group) comprise the electron transport chain? |
NADH + 2 e- Flavoprotein (flavin mononucleotide)
(FADH2 + e- can start the chain here instead of NADH above)
Iron-sulfer protein Ubiquinone Cytochromes (heme) Oxygen Water |
|
|
NADH donates how many electrons for oxygen reduction? FADH donates how many electrons for oxygen reduction? Which electron donor provides less energy for ATP synthesis? |
2 2 FADH (about 1/3 less energy) |
|
|
Smallest molecular rotary motor known in nature? |
ATP synthase |
|
|
How does the mitochondrion couple electron transport and energy release to ATP synthesis? |
Chemiosmosis |
|
|
ATP synthase |
the enzyme that makes ATP from ADP and inorganic phosphate |
|
|
The power source for ATP synthase |
A difference in the concentration of H+ on opposite sides of the inner mitochondrial membrane. |
|
|
Chemiosmosis |
Energy stored in the form of a hydrogen ion gradient across a membrane is used to drive cellular work such as synthesis of ATP (flow of H+ across a membrane).
In general terms, an energy-coupling mechanism that uses energy stored in the form of an H+ gradient across a membrane to drive cellular work. |
|
|
Proton-motive force |
The H+ gradient that results from the spatial arrangement of electron carriers in such a way that H+ is accepted from the mitochondrial matrix and deposited in the intermembrane space. |
|
|
During respiration, most energy flows in this sequence? |
glucose NADH electron transport chain proton-motive force ATP |
|
|
Each NADH that transfers a pair of electrons from glucose to the electron transport chain contributes enough to the proton-motive force to generate how much ATP? |
Max 3 ATP |
|
|
How much H+ must reenter the mitochondrial matrix via ATP synthase to generate 1 ATP? |
4 H+ |
|
|
How much ATp can a single molecule of NADH generate? |
2.5 |
|
|
1 FADH can generate how much ATP? |
1.5 |
|
|
3 factors that make ATP production unpredictable (variable)? |
1. Phosphorylation and the redox reaction are not directly coupled to each other, so the ratio of the number of NADH molecules to the number of ATp molecules is not a whole number
2. The ATp yield varies slightly depending on the type of shuttle used to transport electrons from the cytosol into the mitochondrion
3. The variability of the use of the proton-motive force generated by the redox reactions of respiration to drive other kinds of work |
|
|
Oxidative phosphorylation yields about how much ATP? |
About 26 or 28 ATP |
|
|
Total ATP yield of cellular respiration? |
About 30 or 32 ATP |
|
|
Total output of cellular respiration? |
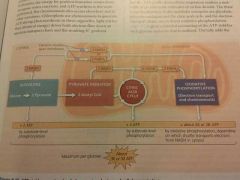
|
|
|
Oxidative Phosphorylation (a) Occurs where (b) Starting products (c) Ending Products |
(a) Mitochondrion (membrane) (b) All end products of other cellular respiration (6-8 NADH, 2-4 FADH2) (c) 26 or 28 ATP (+4 from other sites) |

