![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
What is on this test? |
c = wavelenth x f calculations E = h x f calculations Knowing the relationships between f, wavelength and energy (what goes up or down) Knowing the comparative f and wavelengths of all the types of waves.) Which waves carry more or less energy than other waves. Being able to select the correct electron configurations for ANY element. Being able to pick the correct orbital notation for ANY element. Explaining how light is created according to the line emission spectrum. |
|
|
The product of the frequency and the wavelength of a wave equals the... |
speed of the wave. |
|
|
If electromagnetic radiation A has a lower frequency than electromagnetic radiation B; then compared to B the wavelength of A is. |
longer |
|
|
The energy of a photon or quantum is related to its |
frequency |
|
|
The Bohr model of the atom was an attempt to explain Hydrogen's |
line-emission spectrum |
|
|
According to the Bohr model of the atom; the single electron of a hydrogen atom circles the nucleus... |
in specific allowed orbits. |
|
|
The lowest energy state of an atom is called its _______. |
ground state |
|
|
If the frequency of light is 2.5 x 10^14 Hz what is the wavelength of the light? |
1.2 x 10-6 m
wavelength = c/f |
|
|
What is the frequency a light source emitting with a wavelength of 2.0 x 10^-8 m? |
1.5 x 10^16 Hz
f = c/wavelength |
|
|
Calculate energy in one photon at a frequency of 1.5 x 10^16 Hz? |
9.9 x 10^-18 J |
|
|
Rank the types of electromagnetic radiation from highest energy to lowest energy? |
gamma, X-ray, ultraviolet, visible, infrared, microwaves, radio |
|
|
Rant the types of radiation from longest wavelength to shortest? |
radio, microwaves, infrared, visible, ultraviolet, X-ray, gamma |
|
|
Which type of electromagnetic radiation travels at the fastest velocity? |
All waves travel at a constant velocity |
|
|
What rule or principle states that electrons will occupy the lowest-energy orbital that can receive it? |
Aufbau Principle |
|
|
What rule or principle states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron? |
Hund's Rule
|
|
|
What type or relationship exists between wavelength and frequency: direct or inverse? |
inverse, because when one goes up the other must decrease OR if one decreases the other must increase. |
|
|
Write the correct orbital notation for nitrogen. |
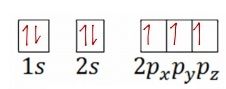
|
|
|
Write the correct orbital notation for potassium. |
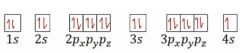
|
|
|
Write the correct orbital notation for phosphorus. |
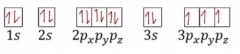
|
|
|
Write the correct orbital notation for iron. |

|
|
|
Write the correct orbital notation for bromine. |
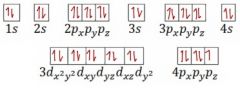
|
|
|
Write the correct orbital notation for antimony (Sb). |
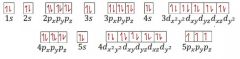
|
|
|
Write the correct electron configuration for the element nitrogen. |

|
|
|
Write the correct electron configuration for potassium. |

|
|
|
Write the correct electron configuration for phosphorus. |

|
|
|
Write the correct electron configuration for bromine. |

|
|
|
Write the correct electron configuration for antimony (Sb). |

|
|
|
Write the correct electron configuration for iron (Fe). |

|
|
|
Write the correct noble gas configuration for silver (Ag). |
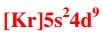
|
|
|
Write the correct noble gas configuration for chlorine. |
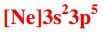
|
|
|
Write the correct noble gas configuration for magnesium. |

|
|
|
Write the correct noble gas configuration for Fr. |

|
|
|
What is the formula for speed of light; c=???? |
c = f x wavelength |
|
|
If electromagnetic radiation A has a higher frequency than electromagnetic radiation B; then compared to B the wavelength of A is |
shorter |
|
|
Is the energy of a photon directly or inversely related to the frequency of the photon. |
Energy and frequency of a photon have a DIRECT relationship...if one goes up, the other goes up. |
|
|
The line-emission spectrum is (the same OR unique) for various light sources. |
it is different (unique) for each light source (we observed this in the lab. |
|
|
When did Bohr hypothesize a photon is light is released:
A) when an electron went from the ground state to the excited state
or
B) when an excited electron fell back to the ground state |
Bohr said a photon of light was emitted when an excited electron fell back to the ground state. |
|
|
The highest energy state of an atom is called its _______. |
excited state |
|
|
If the frequency of light is 1.4 x 10^14 Hz what is the wavelength of the light? |
2.1 x 10^-6 m |
|
|
What is the frequency a light source emitting with a wavelength of 1.6 x 10^-9 m? |
1.8 x 10^17 Hz |
|
|
How much energy is in one photon at a frequency of 3.5 x 10^15 Hz (or s-1)? |
2.3 x 10^-18 J |
|
|
Which travels faster, gamma rays or microwaves? |
Neither; both travel at the speed of light. (3.0x10^8 m/s) |
|
|
What rule or principle states that electrons will occupy the lowest-energy orbital that can receive it? |
Aufbau Principle
|
|
|
What rule or principle states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron? |
Hund's Rule
|
|
|
Write the correct noble gas configuration for Br. |
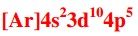
|
|
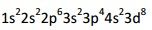
What is wrong with the electron configuration pictured?
|
The 3p orbital should fill with 6 electrons before beginning to fill the 4s orbital. |
|
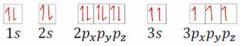
What is wrong with the electron configuration pictured? |
Both electrons in the 3s orbital should have opposite spins (one up and one down). |
|

What is wrong with the electron configuration pictured? |
You must fill each 2p orbital with one up spin (or down spin) before adding a second electron to any 2p orbital. |
|
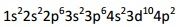
What electron is represented by the electron configuration pictured? |
germanium |
|

What electron is represented by the electron configuration pictured? |
nickle |
|
|
What is the frequency of visible light that has a wavelength of 544 nm? |
5.5 x 10^14 Hz
(use 3.0X10^17 nm/s for the speed of light since your problem used nm). |

