![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
Planck's Constant is
given by what variable? |
h |
|
|
What is the value
of Planck's Constant? |

(6.63 x 10-34) |
|
|
What form of EMR is
below red light on the electromagnetic spectrum? |
Infrared |
|
|
The distance between
adjacent points on a wave. |
wavelength |
|
|
The possible forms
of radiation given off as an electron drops from excited state to ground. |
Electromagnetic Radiation |
|
|
Columns on the
periodic table are called? |
Groups or families |
|
|
A number associated
with an electron to show its location in the electron cloud. |
Quantum Number |
|
|
A packet of energy
given off when electrons move from excited back to ground state |
Photon |
|
|
The state of an
electron that has absorbed energy. |
Excited state |
|
|
A mathematical
number used to make the frequency of a wave equal to it's energy. |
Planck's Constant |
|
|
The relationship
between energy and frequency of a photon is ______________. |
Direct Relationship |
|
|
All of the waves that
are produced when an electron drops energy levels. |
Electromagnetic Radiation |
|
|
What form of electromagnetic
radiation would have the longest wavelength? |
Radio Waves |
|
|
Atoms that have
filled s & p orbitals have ___ electrons in the main energy level. |
8 (eight) |
|
|
Shorthand notation
uses brackets around the symbol of a _________________. |
noble gas |
|
|
What is the
common characteristic shown by all waves in the EMS? |
They all move at the same speed. |
|
|
The change that
occurs when an electron goes from ground to excited state is: |
The electron absorbs energy. |
|
|
An optical instrument
that measures light into it's component wavelengths is called: |
spectroscope |
|
|
The type of spectrum
used to "fingerprint" elements: |
Bright-Line or Emission Spectrum |
|
|
The region of space
where it is likely to find an electron. |
Orbital |
|
|
The term given to
the outermost s & p orbitals of any atom is: |
Valence Electrons |
|
|
The shape of the
orbitals surrounding a nucleus are given by which part of the quantum number? |
Orbital Quantum Number |
|
|
The frequency of a
photon is related to it's: |
Energy |
|
|
The type of radiation
moving at the speed of light given off in an atomic explosion is called: |
Gamma Rays |
|
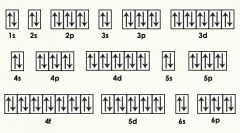
What term?
|
Orbital Diagram |
|

What term?
|
Lewis Dot Notation |
|
|
This states that that each p or d sub-level receive one electron before pairing up? |
Hund's Rule |
|
|
This states that an
orbital can hold no more than 2 e- that must spin in opposite directions. |
Pauli Exclusion Principle |
|
|
This states that
electrons will occupy the lowest possible energy level available. |
Aufbau Principle |
|
|
The type of spectrum
produced when white light is passed through a clear colored medium |
Absorption |
|
|
What type of spectrum
is produced when sunlight is passed through a prism? |
Continuous Spectrum |
|

What term?
|
Shorthand/Noble Gas Notation |
|
|
How many electrons
would be represented in the valence of Oxygen which is in group 16. |
6 (six) |
|
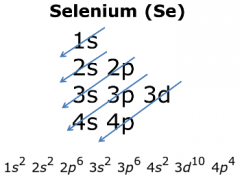
What term? |
Electron Configuration |
|
|
The number of waves that pass a point in a second |
Frequency |
|
|
What color in the visible light spectrum has the longest wavelength? |
Red |
|
|
What color in the visible light spectrum has the highest frequency? |
Violet |
|
|
The part of the quantum number which shows the orientation of the orbital in three dimensions. |
Magnetic Quantum Number |
|
|
The part of the quantum number that shows the spin of the electrons in the orbital or sub-level. |
Spin Quantum Number |
|
|
What are the four orbital quantum numbers? |
s, p, d, f |

