![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
What is an Electrons' charge? |
NEGATIVELY CHARGED |
|
|
Electrons (size)?? |
VERY SMALL |
|
|
Where are ELECTRONS found? |
They are found outside tbe nucleus in the ELECTRON CLOUD. |
|
|
Where is the electron most likey found??? |
ORBITALS |
|
|
ORBITALS are within.... |
The energy levels. |
|
|
ORBITALS are indentified as... |
🔲 or letters |
|
|
ORBITAL SHAPES (S) |
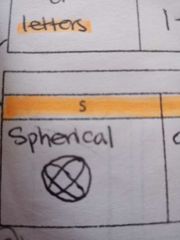
Spherical |
|
|
ORBITAL SHAPES(P) |
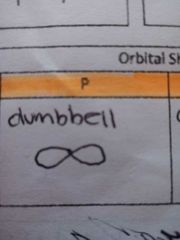
Dumbbell |
|
|
ORBITAL SHAPES(D) |
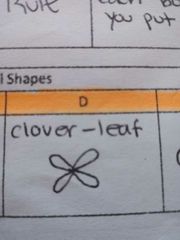
Clover-leaf |
|
|
ORBITAL SHAPES(F) |
Complex |
|
|
THE RULES Aufbow Principle |
You MUST enter the lowest energy level first. |
|
|
THE RULES
Pauli Exclusion Princliple |
There can be a max of 2 electrons per orbital. |
|
|
THE RULES
Hund's Rule |
You must put one arrow in each box before you put 2 |
|

Identify |

|
|
|
Where is the electron most llikely moving? |
Energy Levels |
|
|
What are energy levels made of?? |
Multiple Orbitals |

