![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
188 Cards in this Set
- Front
- Back
|
Disposal of Lab Waste
|
Slides: go into slide disposal pail
Glass waste box: all chipped or broken glassware goes here Pipettes: go into disposal box, NOT garbage |
|
|
Science is a systematic process dealing with questions that can be answered.....
|
empirically, through the use of observations, experience, or experiments
|
|
|
Scientific Method
|
Observation → Hypothesis → Prediction → Experiment → Conclusions (and back to hypothesis if conclusions cannot be met)
|
|
|
Hypothesis
|
A question formulated based on previously obtained facts or observations
|
|
|
A good hypothesis must be...
|
Falsifiable, it can be tested in a way that can lead to evidence that does not support the hypothesis
|
|
|
Prediction
|
"if..then" statement, how experiments are designed
|
|
|
Factors being investigated must be...
|
Measurable
|
|
|
Control
|
the unaffected group in an experiment, which is used to measure outcomes of experiments
|
|
|
Dependent Variable
|
the variable being tested or measured
|
|
|
Independent Variable
|
the variable being changed or controlled by the investigator
|
|
|
Conclusions
|
can be drawn after a hypothesis has been tested and proven or disproven
|
|
|
Theory
|
not just a possible explanation, but a conclusion based on substantial evidence that the scientific community has accepted to be true
|
|
|
Beakers
|
are used to hold or heat large volumes of liquids, they do not measure volume accurately
|
|
|
Graduated Cylinders
|
used to measure large volumes of liquid, and are more accurate than beakers for volumetric measurements
|
|
|
Pipettes
|
used to measure small volumes of liquid (0-10mL)
|
|
|
Types of Pipettes
|
Volumetric: designed to dispense a single volume only, with very high accuracy
Serological: graduation marks that continue to the tip Mohr: graduation marks that stop before the tip |
|
|
Proper Pipette Use
|
never let the fluid enter the pump or the bulb
hold it upright, do not tilt |
|
|
Meniscus
|
portion of a fluid's surface that has a concave appearance, caused by surface tension
*Look at the bottom of the meniscus for accurate measuring* |
|
|
Calculating Percent Error
|
% Error = (Measured Volume - Theoretical Volume)/
(Theoretical Volume) x 100 |
|
|
Spectrophotometry
|
used to measure the amount of light that is absorbed by (on a scale 0-2) or transmitted through (0-100%) a sample, at particular wavelengths of light
The absorbance of a solution is directly proportional to the concentration of the molecules in the solution visible light vs UV |
|
|
Using the Spectrophotometer
|
Blank using water, then test sample, then record data and blank with water again, repeating this process
|
|
|
Absorption Spectrum
|
pattern of light absorption over a range of wavelengths
can help determine the peak absorbance, aka maximum wavelength Experiment 1: peak absorbance of bromophenol blue is 580-600 nm |
|
|
Standard Curve
|
of known concentrations of samples against their absorbances
|
|
|
How to Calculate Unknown (Experiment 1)
|
To figure out unknown, go to absorption value and trace it to the line (standard curve) and drop down, until you hit the concentration on x axis, allowing you to figure out the unknown based on that x value
|
|
|
Components of a Microscope
|
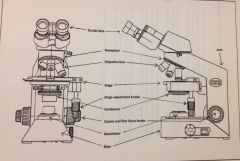
for practical
|
|
|
Knobs on Microscope and Proper Usage
|
Coarse Adjustment: larger knob, use at low magnification, (4x - 10x)
Fine Adjustment: smaller knob, use at higher magnifications (40x - 100x) Using coarse adjust on higher magnifications will result in broken slides |
|
|
Handling the microscope
|
hold with two hands, one on base and one on arm, place it so the arm is facing away from you
|
|
|
Ocular Lenses
|
eyepiece, has 10x magnification
|
|
|
Objective Lenses
|
provide most of the magnification, work with the ocular (4x-100x)
|
|
|
Revolving Nosepiece
|
rotates to desired objective lens
|
|
|
Stage
|
flat platform, where slides are places
|
|
|
Condenser
|
light source under stage, not involved in magnification, but helps concentrate light from illuminator (light source)
|
|
|
Total Magnification
|
ocular lens (10x) times objective (4x-100x) = ___x
|
|
|
Immersion Oil
|
used for 100x, it helps refract/bend the light so that you can see the image at this high magnification
has refractive index similar to glass which allows greater resolution at higher magnifications |
|
|
Calculating Unknown Field of View
|

Ex:
(4x/10x)(4mm) = 1.6mm at 10x, convert to micro |
|
|
Letters under microscope
|
flipped from a d to a p
as you move the slide to left, but as seen through the oculars it moves to the right, etc. |
|
|
Standard units
|
mass: g
length: m volume: L |
|
|
Magnification
|
the number times larger an image appears than its real size
|
|
|
Resolution
|
the effectiveness by which two points can be perceived as distinct
|
|
|
Contrast
|
difference between light and dark areas
higher contrast = more difference |
|
|
Refraction
|
bending of light as it passes from one medium to another
|
|
|
Index of refraction
|
physical property defined by the speed of light in a vacuum divided by the speed of light in the medium
|
|
|
Relationship between Depth of Field and Magnification
|
As magnification increases, depth of field decreases
In order to view the specimen at higher magnifications, either the specimen must become thinner or the field of view must be carefully adjusted up and down to get an accurate idea of its structure |
|
|
Practice Unit Conversion and Dimensional Analysis
|
kilo: 10^3
centi: 10^-2 milli: 10^-3 micro: 10^-6 nano: 10^-9 pico: 10^-12 |
|
|
Dissecting Microscope
|
used to observe small organisms, provides a 3D view at relatively lower magnifications
|
|
|
Compound Microscope
|
used at greater magnifications, used to view thinly sliced things
used in lab |
|
|
Electron Microscope
|
use electromagnets rather than lenses for magnification
Disadvantage: have to kill specimen to use it, where as light microscopy can look at living cells |
|
|
Scanning Electron Microscope
|
used to look at surface of cells, like a dissecting microscope
|
|
|
Transmission Electron Microscope
|
a high resolution, 2D views of thinly sliced things, like the compound microscope
|
|
|
Rotifers
|

Left: shelled
Right: non-shelled |
|
|
Cell
|
smallest unit of life, can perform basic life functions
Function: 1. obtain energy 2. reproduce/replicate |
|
|
Prokaryotic Cells
|
not enclosed by a membrane
no nucleus, has a nucleoid where DNA is stored no membrane bound organelles smaller than eukaryotic cells (1-10 micrometers) divide by binary fission mostly single-celled |
|
|
Eukaryotic Cells
|
is enclosed by a nucleus with DNA in nucleus (more complex DNA material)
membrane bound organelles larger than eukaryotic cells (10-100 micrometers) divide by mitosis single celled and multicellular |
|
|
Cytosol
|
DNA of prokaryotes is suspended in the cell's aqueous interior
where a cell's metabolic processes occur |
|
|
Protists
|
microscopic inhabitants of pond water
|
|
|
Cell Wall/Membrane Composition of Various Cells
|
Bacteria: peptidoglycan
Plants: cellulose Fungi: chitin |
|
|
Organelles
|
only eukaryotes possess these, additional membrane-bound compartments
|
|
|
Surface Area to Volume Ratio
|
puts a limit on cell size
As size increases, the ratio decreases, leading to harder transport of nutrients How to improve it: multiple nuclei, or larger nuclei |
|
|
Organisms that have contractile vacuoles
|
amoeba, paramecium, and certain fungi
|
|
|
Endosymbiont Theory
|
explains how eukaryotic cells could have obtained double membranes (mitochondria and chloroplasts)
a larger cell engulfed a smaller cell, creating a double membrane evolved from a endosymbiotic relationship between two prokaryotes |
|
|
Cytoplasmic Streaming
|
circular flow of cytoplasm in cell (can be seen in elodea)
|
|
|
Central Vacuole
|
can reduce volume within cells
|
|
|
Lactobacillus
|
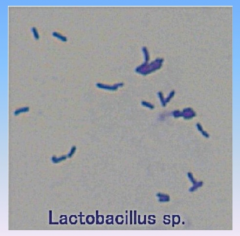
- prokaryote (bacterium)
- produces yogurt |
|
|
Cyanobacteria
|

- photosynthetic prokaryote
- basis of endosymbiont process |
|
|
Elodea
|

- aquatic plant
- can see cytoplasmic steaming - chloroplasts in cytoplasm - cell wall made of cellulose - have large central vacuoles to maintain SA-volume ratio |
|
|
Relating Cyanobacteria and Elodea
|
- both are photosynthetic plants
but as seen with cyanobacteria, photosynthesis does not always require chloroplasts |
|
|
Onion
|
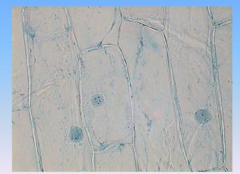
- chloroplasts are absent because they are underground
- you can see cell wall, and nucleus |
|
|
Potato
|
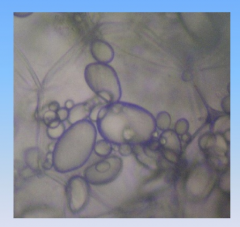
- stored food energy as starch
- starch stored in organelles called amloplasts |
|
|
Amoebae
|

- protists
- no cell wall - cytoplasmic streaming, for food and movement - will move away from light - pseudopodia |
|
|
Paramecium
|
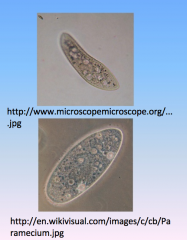
- protists
- use cilia for movement - cytoplasmic streaming for nutrient uptake |
|
|
Hypermastigotes
|
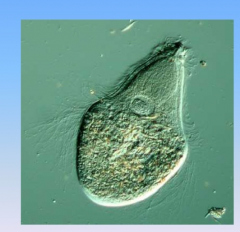
- protist
- found in guts of termites (symbiotic relationship) - digest wood - spirochetes are the method for movement |
|
|
Calculating Length of Cells
|
length of cell = (diameter of fov)/(estimated # of cells in diameter)
|
|
|
Diffusion
|
a random movement toward a state of equilibrium, a substance moves from high to low concentration
any substance can diffuse down the concentration gradient, eventually reaching a dynamic equilibrium |
|
|
Osmosis
|
diffusion of water across a semi-permeable membrane that it may cross but a solute cannot
moves from low solute concentration to higher solute concentration down its concentration gradient |
|
|
Hypertonic
|
high solute concentration, water flows out of them
shrink, too little water |
|
|
Hypotonic
|
low solute concentration, water will flow into them
burst, too much water |
|
|
Isotonic
|
same solute concentration, no net movement
|
|
|
Passive modes of transport
|
diffusion and osmosis, no energy required, move down concentration gradients
|
|
|
Diffusion and Osmosis are important for...
|
proper water balance in a cell
|
|
|
An animal cell in pure water
|
could burst, hypotonic environment
|
|
|
Dealing with certain 'tonic' environments
|
OSMOSIS
animal cells: too much water, bursts plants: cell wall stops water intake protists: have contractile vacuoles to expel excess water |
|
|
Hot and Cold Diffusion Experiment
|
the dye diffused quicker in the hot water because of the quicker particle movement associated with warmer temperatures
|
|
|
Dialysis Diffusion Experiment
|
the sucrose inside the distilled water diffused out of the bag to go from high to lower concentration
if it was distilled water in a bag in sucrose, the water would leave the tube and try to enter the beaker of the sucrose solution |
|
|
pH
|
measure of the concentration of hydrogen ions (protons) in a solution
acidic- more H basic- less H |
|
|
Importance of pH
|
cells need to maintain a certain pH in order to survive
|
|
|
Why can most cells only live in a certain pH range?
|
Cells are typically charged and a different pH range than what a cell is inclined to can change the distribution of charges to the cells (because pH involves the presence or absences of certain amounts of H ions). This would ultimately alter or destroy the cell and its functions.
|
|
|
Which organisms can survive better in changing pH conditions?
|
A free-living cell would survive better than a symbiotic cell because free-living cells often have to adapt to changing environmental conditions whereas symbiotic cells live in closed off environments that do not encounter many environmental changes. An example of a free-living cell would be paramecium. An example of a symbiotic cell is a hypermastigote.
|
|
|
DNA
|
- deoxyribose nucleic acid
- exists as a double stranded, coiled molecule - part of a chromosome - heritable - information storage to make all of a cell's protein molecules - each strand is a polymer composed of nucleotides with adjacent nucleotides joined together by covalent bonds - negatively charged, making it hydrophilic and soluble in water |
|
|
DNA backbone
|
5 C sugar and phosphate backbone with a nitrogenous base connected to the first carbon on the sugar
|
|
|
Phosphodiester bonds
|
the deoxyribose of one nucleotide connected to the negatively charged phosphate group
|
|
|
Hydrogen bonds
|
the two stands are held together by hydrogen bonds between the nitrogen congaing nucleotides, which lie side by side in the double helix
|
|
|
Purine
|
- 2 rings
- adenine and guanine |
|
|
Pyrimidine
|
- 1 ring
- cytosine and thymine |
|
|
The base pair bonding
|
A to T - 2 H bonds
C to G - 3 H bonds (3 rings in total, giving uniform diameter) |
|
|
DNA reproduction
|
complementary base-pairing
|
|
|
Polynucleotide
|
many nucleotides paired together (made of a nitrogenous base, sugar, and phosphate)
|
|
|
How to write a DNA sequence
|
5' 3'
3' 5' match up the complementary base pairs |
|
|
DNA code
|
is redundant, or degenerate
has 64 different code words (4^3) |
|
|
Isopropanol
|
since it is hydrophobic and non polar, while DNA is hydrophilic and polar, the isopropanol will precipitate the DNA
|
|
|
Salt
|
used to neutralize the DNA, since salt is positively charged, while DNA is negatively charged, this prevents the individual DNA strands from repelling each other
|
|
|
Baking Soda
|
maintains pH (a buffer) for the DNA
|
|
|
Shampoo
|
breaks open the cells and dissolves the membrane
|
|
|
UV Spectrophotometer
|
measures absorbance of UV light, can be used to determine amount of DNA in a solution (DNA bases absorb UV light)
OD = nm |
|
|
Peak Absorbances for DNA, RNA, and Protein
|
DNA and RNA: OD260
Protein: OD280 |
|
|
How to estimate amount of DNA
|
by multiplying the OD260 by 50 to get micrograms of DNA
is actually an overestimate, because it is a mixture of DNA, RNA, and protein |
|
|
How to estimate purity
|
OD260 / OD280
|
|
|
Why keep DNA cold?
|
keeping it cold prevents enzymes from coming into the cells and "chopping" it up
|
|
|
Layers in cuvette during strawberry DNA experiment
|
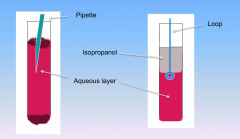
DNA is in the aqueous layer (extract at bottom of isopropanol and top of aqueous layer with loop)
Alcohol is in the isopropanol layer |
|
|
Overexposure to UV light
|
can cause DNA to change its coding, your skin cells cannot absorb light past the peak absorbance, and anything more is overexposure and harmful
|
|
|
Gene
|
hereditary units, consists of a sequence of DNA, codes for a particular trait
one set of genes is inherited from each parent |
|
|
Locus
|
a specific location that DNA occupies on the chromosome
|
|
|
Alleles
|
different versions of genes, result from different base pairing sequences in DNA, alternative expressions of traits; dominant or recessive
individuals with different alleles will have a different number and arrangement of recognition sites for restriction enzymes |
|
|
Are there the same two sequences of DNA between individuals?
|
No, no individual can have the same set of DNA as another person, everyone has different variations of their nucleotides.
|
|
|
Changes in DNA structure
|
can be caused by mutations, during errors of DNA replication, or environmental agents like UV light
|
|
|
How to visualize differences in DNA sequences
|
by the use of restriction enzymes
|
|
|
Restriction Enzymes
|
- are produced by certain bacteria
- they cut at particular recognition sites as defense mechanism, cutting up invading viral DNA - used in DNA fingerprinting |
|
|
Product of Restriction Enzymes
|
sticky and cohesive ends
|
|
|
Micropipette
|
- measures micro liters
- used for DNA, RNA, and protein - for the pipetteman, look at the hashmarks under the numbers to be sure of exact amount (you can tell by if they aren't centered) |
|
|
DNA Fingerprinting
|
analysis of DNA fragments of different sizes through electrophoresis
- provides an accurate, unambiguous identification of the source of the DNA samples - can be used to compare samples of DNA and their cut sites from restriction enzymes |
|
|
Agarose solution
|
- TBE powder mixed with a certain amount of agarose
- heated up in microwave, when it looks clear it is done - is the gel that makes the mold inside the box, the comb is placed in it to make wells can figure what % of solution you need by m1v1=m2v2 or (weight)/(volume) |
|
|
Electrophoresis
|
a technique used to separate molecules based on charge and size
an electrical current is applied to a gel matrix (agarose) used to separate DNA, RNA, and protein |
|
|
Components of Gel Electrophoresis Machine
|
power supply: emits electrical current
buffer: carries electrical current comb: creates wells in gel gel: agarose |
|
|
Agarose and Pore Size
|
high agarose concentration, small pores
low agarose concentration, larger pores |
|
|
Separation by charge
|
since DNA has a negative charge (on the phosphate group), it will migrate to the positive pole in the apparatus
|
|
|
Separation by size
|
smaller molecules travel faster than larger ones because they can slip through the pores better
|
|
|
How to calculate % agarose
|
g agarose / ml buffer
|
|
|
Ethidium Bromide
|
used to stain the gel to be viewed in the UV light box
|
|
|
Test Tubes and their purpose
|
small: for spec
medium: centrifuge large: freezing and boiling enzyme |
|
|
Why should the potato be put on ice?
|
to prevent from enzyme degradation and early reactions
|
|
|
Reaction rate vs concentration
|
directly proportional, higher concentration, faster reaction rate
|
|
|
Reaction rate
|
is not constant, its slope steepest at first and declines over time
|
|
|
Initial rate
|
found by drawing a line on the graph, not a trend line
increases on increasing concentration |
|
|
How to calculate initial rate
|
(y2 - y1)/(x2 - x1)
|
|
|
Room Temp vs 0 degree Reaction
|
room temp reacts better
|
|
|
Boiling vs Freezing Reaction
|
freezing reacted better than boiling
possibly because of denaturing |
|
|
Shaking the tube in enzyme catalysis
|
will speed up the reaction
|
|
|
Adding more oxygen in enzyme catalysis
|
reactions will occur more rapidly
|
|
|
Polyphenoloxidase
|
found in potatoes
|
|
|
Role of enzymes
|
can speed up reactions in the body
|
|
|
Heat catalyst vs Enzyme catalyst
|
heat catalysts have a limit, too much heat will denature the proteins
|
|
|
ATP
|
without ATP, a cell will die, and cellular respiration must be occurring constantly
|
|
|
Glycolysis
|
first phase for cellular respiration and fermentation
is anaerobic, doesn't require or use oxygen occurs in cytosol |
|
|
Fermentation
|
doesn't require oxygen, utilized by yeast, bacteria cells and muscle cells
produces CO2, lactic acid or alcohol |
|
|
Cellular Respiration
|
requires oxygen for the last process, The ETC. Produces co2 and ATP
|
|
|
Yeast Lab Reagents
|
the three carbohydrate solutions mixed with 0.5g yeast
they were put in fridge or in a oven or in room temp |
|
|
The Carbohydrate Difference
|
glucose is the best because it is a monosaccharide and therefore is composed of less and has less bonds to break as it is being metabolized
sucrose was a disaccharide and starch a poly |
|
|
Temperature and Fermentation
|
37 degrees was optimal conditions and 0 degrees contributed to zero CO2 production
|
|
|
DPIP
|
used to asses the oxidation of succinate
will turn from colorless to blue when it accepts the electrons from succinate, making fumarate its role is to serve as the electron acceptor of H ions during the redox reaction, going from oxidized to reduced state a reducing agent (takes something from oxidized into reduced) |
|
|
FAD
|
depletes hydrogen ions succinate to make fumarate, like DPIP
|
|
|
Reagents in Respiration
|
buffer: preserved the pH
DPIP: to show the oxidation and serve as the electron acceptor Mitochondrial Suspension: where the respiration takes place, necessary for process to be observed Succinate: required for the oxidation into fumarate to occur, helped by DPIP |
|
|
Importance of Succinate and Fumarate
|
without succinate being oxidized into fumarate, the entirety of cellular respiration wouldn't occur, it is needed to continue on with the cycle
|
|
|
Initial Reaction Rate Calculation
|
(% final - % initial)/ (time final - time initial)
|
|
|
Redox Reaction
|
oxidation: loss/transfer of electrons
reduced: gain of electrons |
|
|
Pigment
|
molecules responsible for capturing solar energy, located in the chloroplasts
|
|
|
Paper chromatography
|
the separation of plant pigments based on their polarity
|
|
|
Polar
|
dissolve and are attracted to other polar molecules
|
|
|
Nonpolar
|
attracted to other non polar molecules in varying degrees
|
|
|
Absorption Spectrum
|
can be used with paper chromatography, shows the absorption pattern for a pigment at a particular wavelength
determined with a spectrophotometer |
|
|
Transmission vs Absorption
|
a certain color will reflect/transmit the color it appears to be, meaning it will not absorb at this set of wavelengths
it will absorb all the colors it is not |
|
|
Front
|
the leading edge of the solvent
discrete pigment bands will be formed from the front, back to the point where pigments were added to the paper |
|
|
Paper Chromatography Methods
|
in lab, used fresh spinach ground up on the paper
Acetone and ether (organic solvents) were poured into the beaker |
|
|
Importance of different pigments
|
Many photosynthetic organisms have a mixture of pigments. In this way organisms can absorb energy from a wider range of wavelengths and obtain more energy
|
|
|
Acetone
|
has polar and non polar characteristics, therefore during the paper chromatography the least polar substances will travel the furthest up the paper and the polar ones will stay to the bottom
|
|
|
Polarity of molecules
|
Most polar: chlorophyll b, travels least
least polar: beta carotene, travels most |
|
|
Order of Polarity (most to least)
|
1. chlorophyll b
2. chlorophyll a 3. xanthophyll 4. beta carotene |
|
|
Peak Absorbance for each pigment
|
Chlorophyll a: 460, 620
Chlorophyll b: 400-460, 640 Beta carotene: 440-480 Xanthophyll: 420-480 Total pigment: 400-440, 620-680 |
|
|
Plants do absorption...
|
through pigments, the colors attract certain wavelengths of light, giving it energy
|
|
|
visible light spectrum
|

|
|
|
Daughter cells
|
each contain one copy of the chromosome, done by eukaryotes in binary fission
|
|
|
Interphase
|
where most of a cell's life is spent, where it helps it grow in size and duplicate its DNA
|
|
|
Somatic cells
|
all body cells except reproductive, uses mitosis
|
|
|
Germ cells
|
reproductive cells, uses meiosis
|
|
|
Mitosis
|
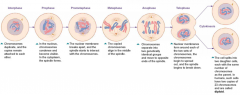
has five phases, results in full set of chromosomes
1. prophase 2. prometaphase 3. metaphase 4. anaphase 5. telophase end result: two daughter cells from one original cell, each daughter cell contains same number of chromosomes from the original cell |
|
|
Meiosis
|
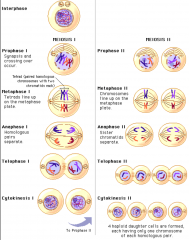
is like a cycle of mitosis twice, meiosis 1 and 2
results in halving the number of chromosomes from original cell, reproductive cells, four daughter cells |
|
|
Crossing over
|
happens during prophase of meiosis 1
results in chromosomes with a different mix of alleles from the parent chromosomes, causes large genetic variation |
|
|
Anaphase vs Interphase
|
anaphase is usually the shortest with interphase being the longest
|
|
|
Diploid
|
full number of chromosomes
2n mitosis |
|
|
Haploid
|
half the number of chromosomes
n meiosis |
|
|
Sordaria fimicola
|
the fungus to show crossing over
|
|
|
% recombinant
|
% recombinant = (recombinant/ recom + nonrecom) x 100
|
|
|
Map Units
|
the unit of measurement used to describe the distance from the centromere to the gene of interest, equal to 1% frequency of recombination
Calculated by: % recombinant x 0.5 = map units |
|
|
Euglena
|
|
|
|
Onion root and the phases
|
|
|
|
Fungi crossing over
|
|
|
|
Elodea leaf
|
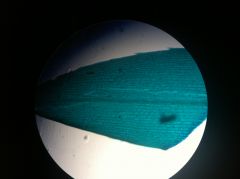
|
|
|
Rotifer
|
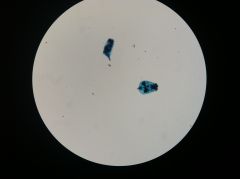
|
|
|
Lactobacillus
|
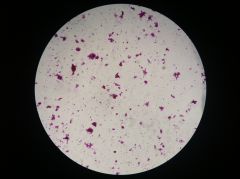
|
|
|
Paramecium
|

|

