![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
Organic Compound
|
Are compounds that contain carbon
|
|
|
Inorganic Compounds
|
Compounds that do not contain carbon
|
|
|
Hydrocarbon
|
Where only molecules are carbon and hydrogen
|
|
|
Charge of hydrocarbons
|
non-polar
|
|
|
Structural Isomers
|
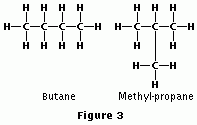
Have different bonding partners, but the same molecular formula
|
|
|
Geometric Isomers
Identify cis and trans |
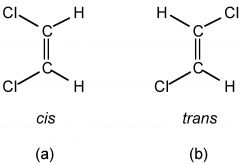
Same bonding partners but spatial arrangement is different around a double bond
Cis: Groups on the same side Trans: Groups on opposite sides |
|
|
Enantimer
|
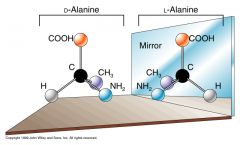
Molecules that are mirror images, usually denoted by a L and D
|
|
|
Amino Groups
-Formula and special function |
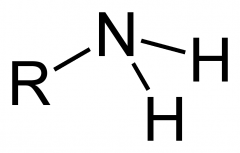
-NH2
-Can act as a base |

