![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
How do we articifially create human protein?
|
Bacteria
|
|
|
Hierarchy of Life
|
Atom
Molecule Organelle Cell Tissue Organ Organ System Organism |
|
|
Four Classes of Biomolecules
|
Proteins
Nucleic Acids Carbohydrates Lipids |
|
|
List of Functional Groups!
|
Alkane/enes
Phenol Amine Alcohol Phosphate Aldehyde Ketone In Lipids - Ester In Proteins - Carboxylic Acids and Amide Very Rare in Biomolecules - Alkynes, Ethers, halides |
|
|
Structure and Function of Proteins
|
Amino Acids with peptide bonding
Function as enzymes and structure |
|
|
Structure and Function of Nucleic Acids
|
Nucleotides with phosphodiester bonding
Function as genetic coding |
|
|
Structure and Function of Carbohydrates
|
Mono/polysaccharides
Function as energy/structure |
|
|
Structure and Function of Lipids
|
Long aliphatic hydrocarbon chains
Function as membrane, storage, signalling |
|
|
Structure and Function of Amino Acids
|
Amino + alpha carbon with 1)carbonyl and 2) R group
Function as basic unit of proteins |
|
|
Structure and Function of Nucleotides
|
Consist of 5-C sugar, a 1-2 ring nitrogenous base, and 1+ phosphate groups.
Function as basic unit of nucleic acids |
|
|
Are proteins static?
|
HECK NO
THEY'RE DYNAMIC/CONSTANTLY IN MOTION |
|
|
Levels of Protein Structure
|
Primary - Number and sequence of Amino Acids
Secondary - Folding that results from intramolecular hydrogen bonding (alpha helix or beta pleated-sheet) Tertiary - Globular structure that results from hydrophobic interactions (hydrogen bonding, salt bridges, disulfide bridges) Quaternary - Bonding of multiple subunits (like amino acids) to form proteins! Same bonding as tertiary, but INTERmolecular. |
|
|
Examples of Polymers and their monomers
|
Polypeptides (proteins) - Monomer: amino acids
Polysaccharides (carbz) - Monomer: monosaccharides Nucleic Acids - Monomer: Nucleotides Lipids - Monomer: Fatty acids and Glycerol |
|
|
Alpha vs Beta Glucose
|

|
|
|
Ribose vs Deoxyribose
|

|
|
|
5 Biochemical Reactions
|
Nucleophilic Substitution (hydrolysis) - Involves the attack of a nucleophile against a carbon with a good leaving group
Elimination - DOUBLE BOND FORMATION Addition (hydration) - It's pretty freakin self-explanatory. Isomerization - Intramolecular SHIFT (movement of pi bonds). NO net gain/loss of atomz Redox - Transfer of electrons from donor to acceptor |
|
|
Describe redox in terms of Oxygen
|
Oxidation is GAIN of oxygen
Reduction if LOSS of oxygen |
|
|
Describe redox in terms of Hydrogen
|
OIL RIG
Oxidation is LOSS of hydrogen Reduction is GAIN of hydrogen |
|
|
Describe redox in terms of electrons
|
OIL RIG
Oxidation is LOSS of electrons Reduction is GAIN of electrons |
|
|
Types of Biochemical Pathways
|
Metabolic
Energy Transfer Signal Transduction |
|
|
Types of Metabolic Pathways
|
Catabolic - Break down of nutrient, generation of energy
Anabolic - MAKING of something |
|
|
How are biochemical pathways regulated?
|
+/- feedback
|
|
|
Traits of Carbohydrates
|
1. General formula is (CH2O)n (twice as many Hydrogens as Oxygen/Carbon)
2. Can be aromatic or aliphatic 3. Polar/hydrophilic 4. Provide fewer calories/gram compared to fats/lipids |
|
|
Calories/Gram of fat, protein, carb, alcohol
|
Fat = 9
Protein = 4 Carbs = 4 Alcohol = 7 |
|
|
Lipophilic is the same as
|
HydroPHOBIC
|
|
|
Aliphatic vs Amphipathic vs Alipathic vs Amphoteric vs Amphiprotic vs Amphiphilic
|
Aliphatic - straight chain; opposite of aromatic
Amphipathic and Amphiphilic - Contains hydrophilic and phobic parts; Alipathic isn't a freaking word. Amphoteric and Amphiprotic are the same - Able to react as an acid or base |
|
|
Traits of lipids
|
1. Long alipathic chains
2. Generally insoluble in water (heavily non-polar) 3. Fat soluble (they're a TYPE of fat) 4. High energy content (9 calories/gram) 5. Also function as membranes, storage, and signalling |
|
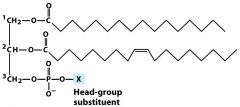
glycerophospholipids are a type of...
|
Lipid (no duh)
|
|
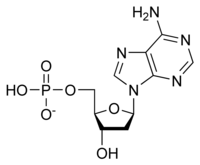
Deoxyadenosine Monophosphate is a type of...
|
Nucleotide
|
|
|
Purines vs Pyrimidines
|
GA are Purines - 2 ring
CTU are Pyrimidines - 1 ring Also for funsies, AT form 2 hydrogen bonds, CG form 3 |
|
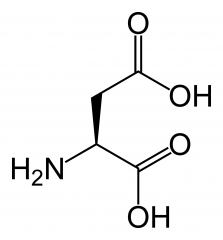
Aspartate is a type of...
|
Amino Acid
(Aspartic Acid) |
|
|
What's alanine?
|
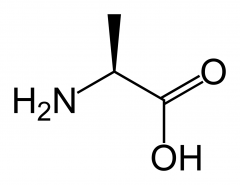
An alpha-amino acid
|
|
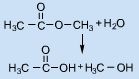
Cleavage of an ester group with water is a ________ of an alcohol by water
|
Nucleophilic Substitution
That was just a dumb way of describing hydrolysis |
|
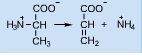
Conversion of alanine to acrylic acid involves the __________ of the elements of ammonia
|
Elimination
|
|
|
Ammonia vs Ammonium
|
Ammonium is a cationic derivative of ammonia
|
|
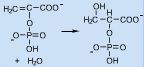
Conversion of phosphoenolpyruvate to 2-phophoglycerate involves the _____________ to a double bond
|
Addition (of water); hydration
|
|
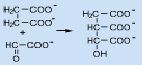
The reaction of succinate with glyoxylate to form isocitrate is an _____________.
|
addition to a carbonyl group
also known as an ALDOL REACTION |
|
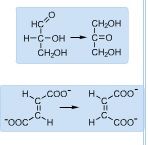
Conversion between gycleraldehyde and dihydroxyacetone as well as the conversion between fumarate and maleate are examples of ________
|
Isomerization
|
|
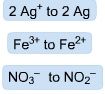
Examples of...
|
Reduction
If you got this wrong, just give up now |
|

Example of...
|
Oxidation
You're not worthy of anything if you missed this |
|
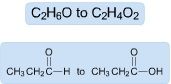
Examples of...
|
Oxidation
Loss of Hydrogens Gain of Oxygens |
|

Example of...
|
Oxidation
Simplify to CH2O vs CO2 PER CARBON, it's a gain of oxygen and loss of hydrogen. |
|

Example of...
|
Reduction
Loss of Oxygen |
|
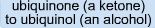
Example of...
|
Reduction
VERY basically involves the gain of a hydrogen |
|
|
Catabolism vs Anabolism
|
Catabolism:
1. Begins with digestion of food (convert large to small) 2. Breaking down releases energy (helps in ATP SYNTHESIS) 3. Exergonic (Spontaneous, release electrons) - example is the reduction of NAD+ to NADH 4. Examples - Glycolysis and fatty acid oxidation Anabolism: 1. Convert small to larger compounds 2. Endergonic (nonspontaneous) - ATP hydrolysis is coupled with endergonic reactions (reactions that require energy) 3. Provide energy by oxidizing NADPH to NADP+ 4. Examples - gluconeogenesis and fatty acid synthesis |
|
|
NADPH to NADP+ is an example of...
|
Oxidation
|
|
|
NAD+ to NADH is an example of...
|
Reduction
|
|
|
How do cells use energy?
|
1. Cellular movement
2. Biosynthesis of more complex molecules (bond FORMATION is endergonic) 3. Active Transport ATP hydrolysis (BREAKING of a bond) is exergonic, so it PRODUCES energy, not uses. |

