![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
233 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Koch's Postulates
|
- agent must be present in all cases of the disease
- agent must be isolated and grown in vitro - disease must be produced when a pure culture is inoculated into a susceptible host - the same agent must be recovered again from the experimentally infected host |
|
|
|
Who developed the 1st attenuated vaccine?
What was the agent? |
Pasteur
rabies virus |
|
|
|
What techniques are used to grow viruses?
|
eggs
cells & tissue cutures genetic material, enzymes |
|
|
|
definition of a virus
|
- genetic material is either DNA or RNA
- simple strx (protein shell surround nucleic acid, with or without envelop) - replicate only in living cells - no energy yielding apparatus - no binary fission - not susceptible to most bacterial antibiotics |
|
|
|
regressive theory vs cellular theory of virus evolution
|
regressive
- degenerate parasites cellular - derived from cellular components |
|
|
|
virion
|
mature virus particle
|
|
|
|
capsid
|
protein coat
|
|
|
|
capsomere
|
capsid subunit
|
|
|
|
nucleocapsid
|
nucleic acid + protein shell
|
|
|
|
peplos
|
lipid envelope
|
|
|
|
peplomer
|
projections on some envelopes
usually glycoproteins |
|
|
|
chemical units refer to
|
polypeptide chains of capsomere
|
|
|
|
a nucleocapsid without a genome is ______
|
a capsid
|
|
|
|
physical properties of viruses
|
Repeating units with spatial regularity.
Small amount of genetic information. Protein subunits assemble spontaneously. Limits variety of morphology: - cubic, binal, complex, helical |
|
|
|
viral shapes
|
Cubic
- Icosahedral - 12 vertices, 30 edges, 20 faces Helical - Spiral - Coiled or rod-shaped Binal - Combination of cubic and helical. - Phages Complex - No distinct morphology. - Poxviruses |
|
|
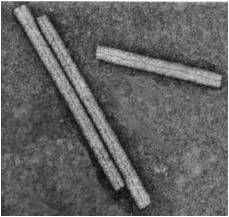
|
helical virus
|
|
|
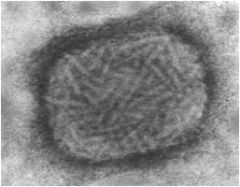
|
complex virus
(no distinct shapes) |
|
|
|
Are enveloped or non-enveloped viruses more pleomorphic?
|
enveloped
|
|
|
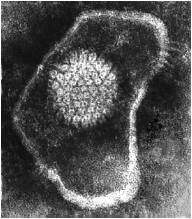
|
enveloped virus
herpesvirus |
|
|
|
infectious genome
|
Purified nucleic acid is infectious
|
|
|
|
viral nucleic acid (excluding retroviruses) is ______.
(haploid or diploid) |
haploid
|
|
|
|
DNA viruses
|
Usually single molecule of ds DNA; some are ss DNA.
Linear or circular 4.5-200kb Differential splicing and open reading frames increases coding capacity. |
|
|
|
RNA viruses
|
Usually ss RNA, few ds RNA.
May be single molecule or segmented. Majority are linear. Considerable 2o structure. + and – sense (most + have poly-A tail and cap) 7.5->20kb |
|
|
|
ss RNA genomes
|
+ve and -ve RNA genomes
Positive – same sense (sequence) as mRNA - Same sequence as RNA - Can be translated by ribosomes Negative – complement (mirror sequence) of mRNA. - Complementary to the RNA - MUST be transcribed to mRNA before translation. |
|
|
|
______ must undergo translation in order to be infectious.
(pos sense/ neg sense) |
neg sense
|
|
|
|
viral proteins
|
Structural or nonstructural.
Protects nucleic acid. Peplomers usually glycoproteins. Surface proteins responsible for attachment to cell surface receptors. Enzymes |
|
|
|
structural vs. non-structural proteins
|
Non-structural are expressed only in the infective state
Structural are carried in the virion |
|
|
|
surface proteins of enveloped vs. non-enveloped viruses
|
Enveloped viruses = surface proteins (peplomere) involved in attachment
- Remove the lipid membrane, and the virus is no longer able to infect b/c can’t attach to target cell - Soaps/ detergent work on this Non-enveloped viruses = surface proteins (capsomere) involved in attachment - Have to use bleach and other stronger treatments to inactivate the virus |
|
|
|
viral lipid is of ______ origin
|
cellular
|
|
|
|
viral lipid and carbohydrate
|
Lipid is of cellular origin.
Envelope mediates attachment, fusion and entry of a virus; released by budding. Carbohydrate usually is component of peplomers; important in antigenicity of the virus. - Virus encodes glycoprotein, inserted in cell membrane (RER). - Piece of cell membrane “stolen” by virus as it leaves the cell |
|
|
|
virus classification
|
Order – virales
Family – viridae Subfamily – virinae Genus – virus Species – often a number Serogroups, strains, variants….. The phylogenetic relationships implied may not be accurate, as origin is unknown. |
|
|
|
how do we classify viruses
(based on what characteristics) |
Structure
- DNA or RNA - Single molecule or pieces - Circular or linear - Enveloped or not - Symmetry? How stable is it in the environment - Easy to inactivate with heat or freezing and thawing How does it replicate & where in the cell it replicates - Some do everything in nucleus except replicate in cytoplasm Biologic properties - Antibodies - Host range How does it spread - From animal to animal or environment to animal Where does it target in the animal? - Skin or lungs or etc. |
|
|
|
_______ & ________ are used to classify viruses
|
morphologic features & chemistry
|
|
|
|
6 features used to classify viruses
|
Properties of the genome.
- Nucleic acid type - N.A. size, sequence Properties of the virion. - Capsid symmetry - Enveloped? - Diameter Properties of proteins. - Size, function, sequence Physical properties. - Stability in varied circumstances Replication strategy. - Entry, transcription, genomic replication, assembly, release - Locale in the cell Biological properties. - Serology, host range, antigenicity, tissue tropism, transmission, vector, epidemiology, etc |
|
|
|
Do DNA or RNA viruses have a higher mutation rate
|
RNA viruses (1/1000)
DNA is only (1/1mil) |
|
|
|
retention of mutation depends on what
|
Selective advantage
Stage of infection |
|
|
|
name 2 ways that mutations can be classified
|
classified according to the type of change in the nucleic acid
classified by their phenotypic expression |
|
|
|
phenotypic mutants
|
Mutation leads to detectable change in the virus; e.g. antigenicity, replication requirements, disease produced…
Plaque morphology mutant - How does it destroy the cell - Does it destroy 1 cell at a time or widespread Host range mutant Temperature sensitive mutant - FIP vaccine (only replicates at cool temp) - No systemic spread Nonsense mutation Deletion mutation Drug resistance mutant |
|
|
|
______ is compensation for lethal mutations between viruses
|
genetic reactivation
|
|
|
|
______ is exchange of nucleic acid sequences between viruses
|
recombination
|
|
|
|
types of recombination
|
Breakage-reunion
- 2 viruses replicating in same cell and cut & paste can be b/w viruses or b/w viral DNA and mRNA - all viruses Template switching - in any virus Reassortment - Only in viruses whose genetic material is in pieces - Influenza (every gene is on a separate piece of genetic material) - Get mixing of segments from 2 different influenza viruses |
|
|
|
what types of viruses can exhibit recombination by reassortment?
|
segmented viruses
|
|
|
|
what types of viruses can exhibit recombination by breakage-reunion?
|
all viruses
|
|
|
|
what types of viruses can exhibit recombination by template switching?
|
all viruses
|
|
|
|
describe template switching
|
recombination event between 2 viruses, not by cutting and pasting, but by switching the template
|
|
|
|
give an example of a recombination event
|
Sometimes FeLk reactivates (provides missing piece) for the virus that was defective and sitting in the cell
If you’re missing a polymerase, I’ll give you mine |
|
|
|
interactions between gene products (proteins)
|
complementation
- exchange of gene products Phenotypic mixing - Transcapsidation - Envelope protein mixing - polyploidy |
|
|
|
describe transcapsidation
|
mixed infection leads to
Hybrid virus in 1st generation Subsequent generations go back to the original b/c the genetic material hasn’t changed |
|
|
|
what types of viruses can exhibit recombination by template switching?
|
all viruses
|
|
|
|
describe template switching
|
recombination event between 2 viruses, not by cutting and pasting, but by switching the template
|
|
|
|
give an example of a recombination event
|
Sometimes FeLk reactivates (provides missing piece) for the virus that was defective and sitting in the cell
If you’re missing a polymerase, I’ll give you mine |
|
|
|
interactions between gene products (proteins)
|
complementation
- exchange of gene products Phenotypic mixing - Transcapsidation - Envelope protein mixing - polyploidy |
|
|
|
describe transcapsidation
|
mixed infection leads to
Hybrid virus in 1st generation Subsequent generations go back to the original b/c the genetic material hasn’t changed |
|
|
|
reassortment is accomplished by antigenic ______
(shift or drift) |
shift
|
|
|
|
the purpose of antigenic shift in nature
|
alteration of antigenicity offers selective advantage
|
|
|
|
antigenic shift vs antigenic drift
|
antigenic drift
- Can mutate w/in itself just by making random mistakes copying its genome - small change antigenic drift - Sometimes segments are exchanged b/w 2 different flu viruses infecting the same cell at the same time - big change - change in whole gene, not just a few nucleotides |
|
|
|
attenuation
|
What was originally a highly lethal virus became a not serious disease
myxomatosis |
|
|
|
define viral epidemiology
|
Study of factors that determine the frequency and distribution of viral diseases.
|
|
|
|
What role does mechanism of transmission play on viral epidemiology?
|
Entry and shedding
Respiratory, enteric, contact, injection, congenital. Influenced by route and duration of shedding, hardiness in environment, existence of reservoirs. |
|
|
|
define incidence
|
# of cases in a time period
|
|
|
|
prevalance
|
# of cases at one time point
|
|
|
|
morbidity vs. mortality
|
morbidity
- % of population that develops disease during an outbreak mortality - % with disease that die |
|
|
|
endemic
|
disease occurs continuously in a population over a period of time
|
|
|
|
epidemic
|
peaks in disease that exceed expected incidence
|
|
|
|
pandemic
|
worldwide epidemic
|
|
|
|
high morbidity suggests that an agent is _______ to spread
(easy/ hard) |
easy
|
|
|
|
modes of transmission
|
Direct – requires physical contact
Indirect – can involve fomites (inanimate objects), airborne (no animal to animal contact, just shared air space) Vector Iatrogenic – by YOU! Nosocomial – in hospital Zoonotic – animals to humans (sometimes the reverse!) Vertical – dam to offspring |
|
|
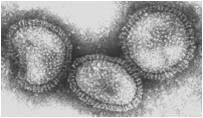
|
influenza
|
|
|
|
highly virulent = _____ period of transmission
(shorter or longer) |
shorter
|
|
|
|
virulence
|
Impacts period of communicability
e.g highly virulent = shorter period of transmissibility |
|
|
|
how does community size affect disease transmissibility
|
Need continuous supply of susceptible individuals.
Population density also important – rate of spread is decreased if the population is spread out, break in chain more likely. |
|
|
|
describe how the population density differs in the transmission of parvo vs. distemper (canine)
|
Parvo in dog doesn’t need a large population to be spread
- It can be shed in environment and then 7 mo later another dog comes around and gets it. Distemper only lasts a few days in order to be able to spread by indirect contact - So normally need direct contact |
|
|
|
persistent infection
|
continual shedding and introduction into a population.
Need smaller population to maintain |
|
|
|
vertical transmission
|
Across placenta
Germ line During birth Breast milk |
|
|
|
do persistent infections need a large or small population? why?
|
Don’t need a large population b/c long shedding from carrier
|
|
|
|
does a wide host range increase or decrease the chance of successful transmission
|
increase
See in arboviruses b/c arthropods feed on many spp. |
|
|
|
how does season affect virus transmission
|
Arboviruses (more arthropods present in fall than spring)
Environmental conditions Management changes, e.g housing indoors |
|
|
|
acute vs persistent infection
|
Acute infection/disease has a relatively rapid course.
- Incubation period of days to weeks. - Clearing of virus within 2-3 weeks of disease onset. Persistent infection may last months or years. |
|
|
|
what are the 4 patterns of disease and give an example of each.
|
acute
- parvo recurrent - callisivirus chronic - FIV slow - prion dz |
|
|
|
what is the importance of persistent infection?
|
May be reactivated and cause acute episode, or cause a late sequela to infection.
May be associated with immunopathological disease. May lead to neoplasia. Important epidemiologically due to recurrence of or continual shedding. |
|
|
|
late complications of acute dz
|
Virus may persist, e.g. brain with CDV;
or effect not detected until later time point, e.g. viruses causing abortions. |
|
|
|
latent infection
|
No virus shedding when latent.
Intermittent recrudescence of latent virus with shedding. Herpesviruses |
|
|
|
chronic infection
|
Virus is always demonstrable.
HIV, FIV |
|
|
|
slow infection
|
Long incubation period followed by slow progressive disease.
“mad cow disease” |
|
|
|
types of persistent infections
|
Late complications of acute infection.
- Virus may persist or effect not detected until later time point Latent infection - No virus shedding when latent. Chronic infection - Virus is always demonstrable. Slow infection - Long incubation period followed by slow progressive disease. |
|
|
|
what are the stages of viral infection (after inoculation) and how many viral particles are present at each stage?
|
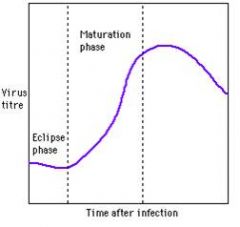
|
eclipse phase
- virion taken into cells quickly after inoculation maturation phase - virions released from cells to infect new cells begins to fall off b/c all the cells have been killed by the virus |
|
|
which type of viral infection can lead to neoplasia?
|
abortive
|
|
|
|
3 types of viral infections
|
productive
- virus will be shed (released from cell & host) abortive - virus enters cell, but can't complete replication - can lead to neoplasia restrictive - virus will grown in certain circumstances but not others (restrictions on where virus can replicate) |
|
|
|
tropism refers to
|
The virus must be able to infect a cell.
|
|
|
|
Know the stages of viral infection starting from attachment
|
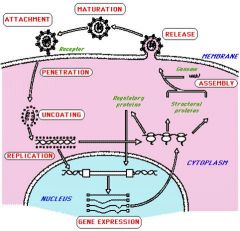
|
|
|
|
virus attachment
|
Chance meeting
Binding mediated via cell receptors Susceptibility determined by the cell receptor Susceptibility + ability of the cell to support virus replication determines the host range |
|
|
|
viral penetration
|
Virus penetrates into the cell
“disappears” Three major mechanisms Endocytosis - nonenveloped and enveloped Fusion - enveloped only Translocation - nonenveloped only Majority fail |
|
|
|
what mechanism of penetration is used by enveloped viruses
|
Endocytosis or Fusion
|
|
|
|
what mechanism of penetration is used by non-enveloped viruses
|
Endocytosis or Translocation
|
|
|
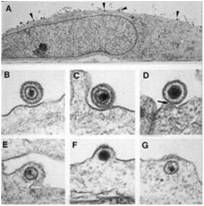
|
endocytosis & fusion
(enveloped virus) |
|
|
|
______ allows transcription to occur
|
virus uncoating
|
|
|
|
virus uncoating
|
Release of n.a.
May occur in cytoplasm or nucleus Allows transcription to occur |
|
|
|
where does virus uncoating occur
|
in cytoplasm or nucleus
|
|
|
|
the product of virus transcription is
|
RNA
|
|
|
|
virus transcription
|
Product is RNA.
ssRNA+ viruses bind directly to ribosomes for translation – do not have to undergo transcription first. ALL other RNA viruses must undergo transcription first. DNA viruses that replicate in the nucleus may use cellular enzymes (DdRp). All others require unique transcriptase. - virus encoded – the cell has NO enzyme that copies RNA into RNA (RdRp) - carried into the cell or immediately translated |
|
|
|
Virus transcription for DNA viruses
|
Transcription is sequential.
- Early, late Important step in regulating gene expression. - Promoter control - termination signal - splicing - rate of degradation “Genetic Economy” - both strands - gene overlap - different ORF - variation in termination and splicing |
|
|
|
Virus Transcription
RNA Viruses |
More complicated; no clear early and late phase.
(-) strand - transcription by virion-associated transcriptase. (+) strand - translation produces virion-associated transcriptase. dsRNA - segmented - each a unique gene; undergo transcription first using virion-associated transcriptase. Retroviruses - reverse transcribed to ds DNA , then mRNA transcribed. |
|
|
|
how are retroviruses transcribed?
|
reverse transcribed to ds DNA , then mRNA transcribed.
|
|
|
|
dsRNA virus transcription
|
segmented
- each a unique gene undergo transcription first using virion-associated transcriptase. |
|
|
|
virus translation
|
mRNA translated by cellular ribosomes; product is protein.
Uses cellular machinery. - Monocistronic vs. polycistronic messages. - Monocistronic – one gene→one protein - For polycistronic messages: Internal initiation of transcription; Polyprotein that is cleaved; or Translate only the first ORF. ONE message → One protein |
|
|
|
monocistronic vs. polycistronic messages
|
Monocistronic
- one gene→one protein polycistronic messages: - Internal initiation of transcription; Polyprotein that is cleaved; or Translate only the first ORF. |
|
|
|
describe how a virus gets genetic economy but still fits the rule of 1 message -> 1 protein
|
Sometimes the virus can fold their single stranded mRNA and get a 2nd product produced
- Internal ribosomal entry site allows the 2nd translation (polycistronic message) but it is seen as monocistronic to the ribosomes Cellular ribosomes recognize only 1 message on a mRNA There may be splicing that occurs but the product is only 1 protein As far as ribosomes are concerned, all are monocistronic But as far as the virus is concerned, it may get several proteins out of 1 piece of mRNA |
|
|
|
Do pos sense RNA viruses tend to be mono or polycistronic?
|
polycistronic
Tend to make a big protein and cut it up |
|
|
|
Do neg sense RNA viruses tend to be mono or polycistronic?
|
monocistronic
Make each strand individually |
|
|
|
describe nested transcription
|
all protein products share a 3' end but they have different 5' ends (start at different points on the strand)
Only unique, 5’-most ORF is translated |
|
|
|
early vs late proteins
|
Early proteins (before genome is copied) are generally enzymes.
Late proteins (after genome is copied) are generally structural. |
|
|
|
how do viruses make a capsid?
|
No cellular machinery to make a capsid
After proteins form, they crystallize together to form a shell |
|
|
|
replication of nucleic acid
|
DNA viruses
- similar to cellular DNA; product and template are DNA. - May use cellular enzymes. RNA viruses – product and template are RNA - MUST use viral enzyme (+) --> full length (-) --> full length (+) (-) -->full length (+) --> full length (-) Retroviruses: RNA --viral enzyme--> dsDNA ---->integrates into cellular DNA --cellular enzyme--> RNA |
|
|
|
how are viruses released from the cell?
|
cell lysis
budding |
|
|
|
for enveloped, how is the membrane acquired
|
membrane acquired by budding through cellular membrane
|
|
|
|
what happens after replication of the genome?
|
Structural proteins synthesized.
Proteins aggregate spontaneously. virus is released by cell lysis or budding. |
|
|
|
when does a virus mature?
|
after release from the cell
|
|
|
|
attachment exploits ______
|
a cellular protein
|
|
|
|
circoviridae
non-enveloped (cookie cutter shaped) |
|
|
|
adenoviridae
Icosahedral nonenveloped virus |
|
|
|
Icosahedral nonenveloped virus
adenoviridae |
|
|
|
Icosahedral nonenveloped virus
adenoviridae |
|
|
|
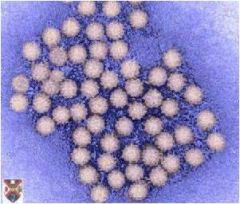
parvoviridae
small, icosahedral capsid non-enveloped |
|
|
|
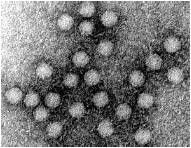
parvoviridae
small, icosahedral capsid non-enveloped |
|
|
|
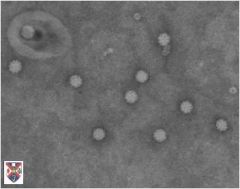
circoviridae
Nonenveloped, icosahedral capsids |
|
|
|
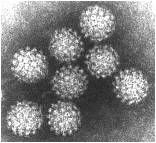
circoviridae
|
|
|
|
papovaviridae
Icosahedral capsid. Nonenveloped |
|
|
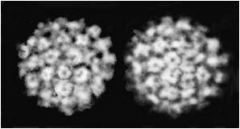
|
Icosahedral capsid.
Nonenveloped papovaviridae |
|
|
|
parvovirinae is associated with what types of animals?
|
vertebrates
|
|
|
|
parvovirus
|
veterinary pathogen
|
|
|
|
erythrovirus
|
human B19
|
|
|
|
aleutian mink disease is associated with what virus
|
ambovirus
(parvovirinae) |
|
|
|
bocavirus
|
Bovine parvovirus and minute virus of canines
human bocavirus – respiratory, gastroenteritis in infants – role unclear |
|
|
|
what parvoviridae are associated with invertebrates
|
Densovirinae – insects, other invertebrates
- Densovirus - Iteravirus - Brevidensovirus - Pefudensovirus |
|
|
|
_______ is the smallest animal virus
|
parvovirus
|
|
|
|
describe parvovirus
|
18-26nm
Icosahedral capsid, 32 capsomers. 3 proteins – VP1-3 No envelope nor enzymes in mature virion |
|
|
|
describe parvovirus's genome
|
ssDNA genome
- 5-5.5kb - Negative sense - Y-shaped hairpins at both ends due to palindromic sequences. 2 major ORF’s Left half – 2 NS proteins Right half – 3 coat proteins |
|
|
|
why do parvoviruses need rapidly dividing cells?
|
b/c they don't produce any enzymes that can push the cell into the cell cycle.
|
|
|
|
parvovirus replication
|
Replicates in nucleus.
Depends completely on host cell enzymes. Replicates in cells of “high mitotic index.” - Bone marrow - Intestinal crypts - Fetus Virus does not encode replication enzymes such as polymerase. Uses cellular DNA polymerase and RNA transcriptase Uses cellular splicing machinery |
|
|
|
where does parvovirus replicate?
|
in the nucleus of rapidly dividing cells (intestinal crypt, bone marrow, fetus)
|
|
|
|
describe parvo replication (ORF/ proteins made)
|
3’ORF encodes VP1-3.
5’ORF encodes NS1-2. - Regulate gene expression. - Function in DNA replication – helicase, endonuclease, nicks cellular DNA to inhibit its replication. VP1 and VP2 formed by alternative splicing of same mRNA. VP3 formed by cleavage of amino acids from VP2. |
|
|
|
parvo genomic replication is primed by _______
|
primed by hairpins.
Complex replication, with circular ds intermediates and concatemers. |
|
|
|
describe parvo entry through replication of genome
|
Virus will attach to cell surface molecule
Magically gets into cell Traverses to nucleus Uncoats and releases its genome Transcription to make mRNA 2 mRNA for 2 genes (structural and non-structural) Differential splicing allows virus to get 2 products from this 1 protein (NS1 & NS2) For structural proteins, they start at different initiation points (VP1 & VP2) Structural protein 3 is produced from further proteolytic processing from the VP2 |
|
|
|
describe the importance of hairpins/ concatemers for parvo replication
|
Hairpins serve as primers for host cell enzymes to start copying the genome
Cut up into individual pieces by viral particles Concatemers (multiple copies of the genome) are formed, cleaved by viral endonuclease |
|
|
|
how does exposure to parvo occur?
|
oronasal exposure
(not ingestion) |
|
|
|
where does parvo replicate
|
in lymphoid tissue
Replicates in cells of high mitotic index - Bone marrow - Intestinal crypts - Fetal tissue (can cause abortion) |
|
|
|
how is parvo shed?
|
Shed in feces (dogs, cats, others), aborted material and semen (swine)
|
|
|
|
describe parvo in dogs (direct result and secondary action)
|
Can get profound leukopenia which is dangerous in itself
Combine that with the damage of the intestinal epithelium and you get a secondary sepsis |
|
|
|
describe parvo disease syndromes of veterinary importance (4)
|
Panleukopenia of cats.
Hemorrhagic enteritis in dogs. Abortion and stillbirth in swine. Aleutian mink disease. |
|
|
|
describe parvo in humans
|
5th disease
slapped cheek aplastic crisis in pt with underlying hemolytic dz fetal infection inclusions in RBC precursors |
|
|
|
feline panleukopenia
|
Parvo
- mucosal destruction - villous atrophy - cerebellar hypoplasia Infects all Felidae (including exotic felids) Profound leukopenia (decreased WBC’s) due to replication in bone marrow. Vomiting, bloody diarrhea, depression. Neonates suffer cerebellar hypoplasia. Spread by direct and indirect contact. Vaccination with killed or modified live vaccines. |
|
|
|
canine parvovirus dz
|
CPV 1 – minute virus of canines – mild disease if any.
- Fetuses and neonates only - Wipes out puppies very rapidly (almost no signs of dz) CPV 2 – a “new” virus. - Emerged in late 70’s worldwide. - All canids susceptible. Similar symptoms to FPV – intestines, bone marrow. Myocarditis in neonatal pups. |
|
|
|
how does parvo infection differ from other enteric viruses
|
Most enteric viruses target mature cells at tip of villus causing malabsorption
Parvo targets crypt cells so no ability to regenerate intestinal epithelium |
|
|
|
describe canine parvovirus vaccinations/ recent mutations
|
Disease primarily occurs when maternal immunity wanes.
Killed and modified live vaccines. - Killed only in utero or just after born - All others get modified live Mutations led to appearance of new strains 2a and 2b in 80’s; recently 2c has emerged. - Reacquired ability to replicate in cats. All strains are not in the vaccine, but they are cross protective. |
|
|
|
swine parvo
|
Swine: fetal tissue.
- Embryonic death, mummification, stillbirth, birth of weak pigs depending on stage of gestationg. Recovered animals may be carriers. Killed and modified live vaccines in gilts. Variation in tropism among species probably related to expression of appropriate cell receptor. Neutralizing antibody important in protection. |
|
|
|
goose & duck parvo
|
Lethal to goslings and Muscovy ducklings –
Hepatitis, myocarditis |
|
|
|
rodent parvo
|
Common in lab colonies.
Fetal death. Subclinical carriers. Affect immune response, confounds research. |
|
|
|
aleutian dz
|
Immune-mediated
Virus not eliminated, immune complexes form. Mink homozygous of pale (“Aleutian”) coat color susceptible. Glomerulonephritis, vasculitis, hepatitis, anemia and death. See bone marrow problems, intestinal problems, and immune system diseases The outlier is aleutian disease b/c of immune complex formation Skin, renal disease, anemia, etc. |
|
|
|
how do you detect parvovirus
|
Usually look for virus itself not by antibody detection b/c lots of animals have antibodies
|
|
|
|
how do you destroy parvovirus
|
Something with detergent will get rid of this (ammonia won’t work) need oxidizing agent
|
|
|
|
parvo dx & tx
|
Symptoms
- Enteric - Identification of virus by EM or ELISA. - Virus isolation, immunofluorescence of tissue (esp fetus). - Serology for antibody detection. tx - Supportive treatment. - Control in veterinary medicine through vaccination. - Disinfectants must have oxidizing agent as active ingredient |
|
|
|
Circoviridae genera
|
Circovirus – porcine, psittacine circoviruses, other avian circoviruses (pigeons, finches, doves among others).
Gyrovirus – chicken anemia virus. Viruses share no antigenic determinants nor DNA homology with one another. |
|
|
|
is circoviridae a high risk for mutation or not?
|
Very little genetic homology so not a high risk of mutations
|
|
|
|
how does circoviridae compare to parvo?
|
Both single stranded DNA
Target rapidly dividing cells The difference is their genome - Smaller genome than parvo - But that genome has 3-4 genes depending on strain |
|
|
|
circoviridae properties
|
Nonenveloped, icosahedral capsids, 17-22nm.
Circular ssDNA, ambisense (porcine, psittacine) or negative sense (chicken), 1.7- 2.3kb. 3-4 ORF’s. Single protein forms capsid Some have apotin which induces apoptosis of T cells. Stable in the environment |
|
|
|
circoviridae replication
|
Occurs in the nucleus.
Similar to parvovirus, probably depends on cellular proteins produced in S phase of cell cycle. Genome replication occurs via rolling circle. Post-translational cleavage may occur. |
|
|
|
where does circoviridae replication occur?
|
in the nucleus
|
|
|
|
describe how the circoviridae genome is copied
|
Circular so different way of copying the genome
Some regions of the genome are the same sequence as the mRNA Some regions are the complement of the mRNA So it has both pos and neg sense (ambisense) |
|
|
|
where does circoviridae target in a bird
|
Rapidly dividing cells in the bird are feather follicles and beak
Also goes for WBC and occ digestive problems |
|
|
|
psittacine beak and feather disease
|
Chronic disease of psittacines characterized by feather dystrophy and loss, beak abnormalities.
PBFDV 1 and 2 (latter in lories) - circoviruses Generally a disease of young birds. Necrotic abnormal feathers. Beak deformities develop later. Pneumonia, enteritis due to secondary infections. Occurs worldwide. Endemic in wild populations. Similar virus in doves in Australia. Targets epithelial cells (dividing cells of basal layer) leading to feather necrosis and abnormal beak formation. Also necrosis of thymus and bursa of Fabricius(!) Shed in feather dander, droppings. Vertical transmission via egg does occur. Carrier state may occur. Inhaled or ingested, spreads systemically. Immunosuppression occurs. - Due to thymus and bursa atrophy. |
|
|
|
porcine vasculitis
|
circovirus
PCV 1 - Widespread in pig populations. - May be a fetal pathogen. PCV 2 - Young pigs with wasting disease syndromes – postweaning multisystemic wasting syndrome (PMWS). - Respiratory disease, pneumonia. - Dermatitis (due to necrotizing vasculitis) and nephropathy – immune-mediated? - Reproductive disease, enteritis, CNS disease. |
|
|
|
wasting syndrome (pigs)
|
Major economic concern
Occurs worldwide Depleted lymphocytes Virus found in lymphocytes, monocytes, macrophages, as well as lung. Concurrent infection with other pathogens Wasting, failure to thrive; enlarged lymph nodes Nursery age piglets. Mortality of 10% and higher. |
|
|
|
chicken anemia virus
|
Gyrovirus (Circoviridae)
Occurs worldwide. Chicks susceptible; resistance increases with age. Transmitted by direct and indirect contact, and through the egg. Viremia develops after exposure. Virus replicates in RBC precursors, thymus (incl T helpers), and spleen. Infection leads to decreased erythropoiesis and anemia, and immunosuppression. Mortality may be as high as 50%, usually less. Diagnose with signs, pathology, and virus isolation; serology can be done. Vaccine available in Germany. |
|
|
|
papovaviridae subfamilies
|
papillomaviridae
polyomaviridae |
|
|
|
is adenoviridae capable of causing oncogenesis in natural infection?
|
no
only seen experimentally |
|
|
|
is papillomaviridae capable of causing oncogenesis in natural infection?
|
yes
|
|
|
|
papillomaviridae vs. polyomaviridae
|
Papillomaviridae
- Cause warts in animals. - Associated with oncogenesis experimentally and in natural infection. - Many groups distinguished antigenically. - Given separate genera designations. Greek letters,(Alphapapillomavirus, Betapapillomavirus, etc.) Or referred to by host. - Many types determined by DNA sequence. Polyomaviridae - One genus - Polyomavirus - Rare disease in humans. - Significant veterinary pathogen. (Pathogen of psittacines.) - Oncogenic only in experimental systems. |
|
|
|
is polyomaviridae capable of causing oncogenesis in natural infection?
|
no
only experimentally |
|
|
|
compare papovaviridae to parvo/ circo/ adenoviridae
|
Spherical (icosahedral) virus
Twice the size of parvo ½ the size of adeno More complex than parvo and circo but still pretty small Circular (covalently closed) genome |
|
|
|
papovaviridae properties
|
Icosahedral capsid.
- Nonenveloped. - 45-55nm diameter. - 72 capsomers. - Three viral proteins make up the capsid of polyoma-, two for papillomavirus. dsDNA - Covalently closed circle. - 5-8kbp. - Genome is divided into early and late regions. - Noncoding elements control transcription and replication. |
|
|
|
early region vs late region of genome
|
Early region
- Those proteins expressed before replication - Proteins used for replicating the virus - Regulatory fxns in transcription, gearing up the cell, etc. - Non-structural proteins (don’t leave the cells with the virus) Late region - Those proteins expressed after the DNA is synthesized - Makes up the protein shell of the virus - Structural proteins |
|
|
|
papovaviruses' replication
|
Replicates in nucleus of cell.
Receptor unknown; may be growth factor receptor. Enters by endocytosis, uncoats in nucleus. Early and late phases of transcription. - Early phase done by cell RNA polymerase. Very complex control of transcription. |
|
|
|
where does papovavirus replicate?
where does it uncoat? |
in the nucleus
in the nucleus |
|
|
|
describe papovaviridae from attachment through replication
|
Attaches to the cell surface (receptor unknown)
Endocytosed into cell Magically translocates into nucleus where it uncoats Early transcription by cellular polymerase (DNA dependent RNA polymerase) Non-protein encoded regions of genome are impt regulators of transcription (steals the host polymerase) When enough of the non-structural proteins are made, switches to transcription of the genome |
|
|
|
polyomavirus replication
|
Early mRNA is transcribed from one strand, while late mRNA is transcribed from the other strand.
Early proteins are T antigens – 2-3 proteins. - Coding regions overlap. - Significant splicing of mRNA. - Alternative splicing leads to different proteins from same ORF. Products activate cellular genes leading to DNA synthesis. |
|
|
|
polyomavirus DNA replication
|
DNA replication
- similar to chromosomal replication. - Depends on cellular enzymes. - Begins at origin, proceeds in both directions. - Early proteins initiate replication and unwind helix. |
|
|
|
polyomavirus late transcription
|
Follows DNA replication.
Structural proteins of capsid. Alternative splicing of mRNA, use of different initiation codons and reading frames produce capsid proteins VP1-3 Transported to nucleus for assembly Release due to cell death. - May be induced. - May be a result of natural turnover. |
|
|
|
papillomavirus has a specific tissue tropism for ______
|
squamous cells
infects basal cells |
|
|
|
how is papillomavirus shed?
|
in exfoliated cells
|
|
|
|
papillomavirus replication
|
Early and late transcription.
Transcription very complex – multiple promoters, complex splicing, regulation of mRNA production. 9-10 ORF’s – 7-8 early genes, 2 late genes. Early proteins induce cellular hyperplasia via early proteins; mass into papillomas. Late transcription and translation occur in terminally differentiated cells. DNA replication similar to polyomavirus. |
|
|
|
why can't we culture papillomavirus?
|
we can't mimic the differentiation process
|
|
|
|
why are papillomaviruses associated with tumors?
|
Virus is promoting the growth of the cell
- In productive infection that is okay b/c cell will be destroyed Genome persists, usually episomally; can integrate into cellular DNA. Early proteins expressed, lead to transformation. Affect growth factors, other cellular factors. Cofactors required for malignancy. Cervical cancer in women; gastric carcinoma in Scottish cattle. |
|
|
|
polyomavirus
|
Rare
BKV - Entry into respiratory tract. - Spreads via blood. - Eventually to urinary tract. - Usually inapparent. JCV - Progressive multifocal leukoencephalopathy. - Complication of certain immunosuppressive or malignancy disorders. |
|
|
|
avian polyomavirus
|
Occurs worldwide.
- First identified in budgies. “budgerigar fledgling disease” - Infects all psittacines, some nonpsittacine, e.g. finches. Virus is shed in droppings, dander, oral secretions; vertical transmission may occur. Disease development is age dependent. - Nestling and juvenile birds most susceptible. - Disease in adults is rare. Virus replicates in wide variety of tissues. - Viremia occurs after entry. - Infects endothelial cells, hepatocytes, renal tubules, monocytes/macrophages. Acute death in birds <2 weeks of age. - Hepatic necrosis - Ascites, hyeropericardium, neurologic signs. Dysplastic feathers in those that develop signs after 2 wks of age. - Symmetrical. - May be the only sign. - Results from damage to blood vessels supplying the feathers no tx but vax available |
|
|
|
papillomavirus disease
|
Enters through skin abrasions.
Infects basal cells; early proteins induce hyperplasia. Usual outcome is benign growth. May persist for months or years, or may regress spontaneously. |
|
|
|
papillomavirus lesions
|
Common warts
- Areas subject to abrasions. Flat or planar warts - children Plantar/palmar warts - Weight-bearing areas Epidermodysplasia verruciformis. - Wide distribution, associated with immunosuppression. Genital warts - Mucosal not cutaneous Laryngeal papillomas - Children. |
|
|
|
what is papillomavirus's role in cancer
|
HPV detected in 93% of cervical cancers.
Viral DNA remains un-integrated. Nonproductive infection. Involves early gene products. Inhibit growth arrest, degrade tumor suppressor protein. |
|
|
|
bovine papillomas
|
Common.
Affects all ages, but highest incidence in calves. Enter through skin abrasions. - Fomites such as halters, nose tongs, posts important in spread. Fibropapillomas or epithelial papillomas depending on virus species. Papillomas persist for months, then spontaneously regress. Associated with gastric and urinary bladder carcinomas in Scottish cattle. - Ingestion of bracken fern contributing factor |
|
|
|
equine papillomas
|
Often around nose and lips.
Regress after several months. Sarcoids are skin tumors that persist for life and are locally invasive. - Several types based on appearance and growth characteristics. Horses are susceptible to infection with some bovine papillomas – may be the cause of sarcoids. |
|
|
|
canine papillomas
|
Usually begin on lips, spread to mucosa, tongue, palate, pharynx.
Regress spontaneously. If block airway, may require surgery. |
|
|
|
papillomas in birds
|
Finches, psittacines.
In finches, papillomas occur on legs. |
|
|
|
the legendary jackelope may be associated with what virus
|
papillomavirus
|
|
|
|
dx, tx, control of polyomavirus
|
Diagnose with virus detection by PCR, serology for antibody.
No treatment Control with management (quarantine, disinfection, screening), and vaccine. only prob is avian, control issue is aviaries |
|
|
|
dx, tx, control of papillomavirus
|
Diagnose by characteristic lesions.
Human medicine – DNA hybridization to identify viral genome. Usually regress on their own; surgical excision when needed. Vaccine? |
|
|
|
adenoviridae properties
|
Icosahedral nonenveloped virus.
- 80-100nm diameter - 240 hexons (capsomers of the faces and edges). - 12 pentons (vertices). - Glycoprotein fibers protrude from each penton – responsible for attachment. Linear dsDNA - 30-38 kbp. - 30-40 genes; ~1/3 encode structural proteins. - Terminal protein covalently attached to 5’end; primes DNA synthesis. - Histone-like proteins present |
|
|
|
mastadendovirus
|
Mammalian viruses.
Many animal species including humans. In people, causes colds, sore throat, upper respiratory infection, gastroenteritis, conjunctivitis; occasionally cystitis and hepatitis (infants). |
|
|
|
atadenovirus
|
ruminant, fowl, possum, snake, other reptiles viruses.
|
|
|
|
aviadenovirus
|
Avian viruses.
Three groups: I – many species of birds; causes hepatitis, bronchitis. II – hemorrhagic enteritis, splenic disease in fowl (turkeys) – Now classified as separate genus: Siadenovirus Egg drop syndrome – fowl; thin-, soft-, or no shell eggs. - May be reclassified as Atadenovirus |
|
|
|
adenovirus pentons
|
Toxic protein
If you take that from the virus and inject it in a cell, it will kill the cell Interferes with cell membrane Fibers attached to pentons |
|
|
|
adenovirus attachment through replication
|
Attach via penton fibers.
Enters by endocytosis; endocytic membrane is ruptured by the pentons toxic activity. Pentons and outer capsid removed Core (consisting of genome and histones) migrates to nucleus, where uncoating, transcription (done by cellular protein) and replication occur |
|
|
|
adenovirus early transcription
|
Done by host cell RNA polymerase.
Early proteins primarily nonstructural. - E1A produced first – transcription activation, incl of cellular genes. - Early proteins induce cell cycle progression (DNA synthesis), protect infected cell from immune response, and induce synthesis of proteins for DNA replication. Functions of early proteins not completely understood. Affect DNA replication, gene expression, host response; transformation. E1A and E1B can transform cells. E3 inhibits apoptosis by Tc Cells and downregulates MHC I expression. |
|
|
|
describe what happens in early transcription of adenovirus
(in general terms) |
Adenoviruses produce proteins in early phase to push cell into cell cycle so the cell will produce those enzymes that the virus needs to replicate
Produces things that interfere with interferon Has the ability to start the ball rolling and make the cell grow due to the virus direction and if the cell isn’t killed (abortive virus) then the cell can continue growing out of control resulting in cancer - Only seen in the lab so far, but vax controversial |
|
|
|
adenovirus late transcription
|
Late transcription occurs after onset of DNA replication.
Late proteins primarily structural. Uses newly synthesized DNA as template for late mRNA. Complex control through promoters and feedback regulation. Extensive splicing of mRNA. |
|
|
|
only viruses that replicate _______ can undergo splicing
|
in the nucleus
|
|
|
|
how does the priming of transcription differ in adeno vs. parvo
|
In parvo, the DNA flipping back on itself is what primes transcription
In this, a protein product of early transcription is what primes the DNA |
|
|
|
adenovirus
products of early transcription |
Viral polymerase
Anti-interferon protein |
|
|
|
adenovirus
DNA replication |
Occurs after early transcription.
Requires viral DNA pol, DNA binding protein and terminal protein. Host factors also required. Proceeds by strand displacement. - no lagging strands - no okasaki fragments Primed by 5’-linked terminal protein. Proceeds from both ends |
|
|
|
how is adenovirus deleterious to the host cell
|
Transcriptase is shifted from making host cell messages to viral messages
- It is creating viral parts not cell parts causes cytolytic infections Affects cell profoundly. - Decreased cell protein synthesis. - Inhibition of cell DNA synthesis. - Toxic penton. - Cell death. |
|
|
|
adenovirus assembly in nucleus
|
Capsomers assembled, then capsid.
NA is packaged. Core proteins added. Accumulate in cell. Released by lysis (no specific pathway for this lysis). |
|
|
|
human adenoviridae dz syndroms
|
Respiratory disease
- Children – acute febrile pharyngitis. - Childhood pneumonias - Acute respiratory disease of military recruits Conjunctivitis – “pink eye.” Acute hemorrhagic cystitis (infants) Gastroenteritis (less commonly – 24 hr bug) Meningoencephalitis – rare. |
|
|
|
1) are respiratory viruses usually enveloped or not?
2) enteric? |
1) enveloped
2) non-enveloped |
|
|
|
veterinary adenoviridae dz syndromes
|
Canine
- Infectious canine hepatitis. - Upper respiratory tract disease (kennel cough). Equine - Upper respiratory tract dz - Serious in immunocompromised foals – pneumonia. Bovine - Respiratory and enteric dz Avian |
|
|
|
how are adenoviridae spread
|
Spread primarily by fecal-oral spread; aerosol for respiratory forms; indirect spread via contact with contaminated objects (“fomites”).
Canine Adenovirus I spread by urine, persists for months in renal tissue. - Impt b/c dogs smell each other’s urine |
|
|
|
canine adenovirus 1
|
Infectious canine hepatitis.
Infects canids; also skunks, bears. Entry through mucosal surfaces (nasal, oral, conjunctival). Replicates at entry site in the epithelia and may spread to blood stream Viremia follows. Infects endothelial cells and parenchymal cells of many tissues, incl. liver. - Usually in liver but can be in brain and other places b/c endothelial cells are everywhere - encephalitis in foxes Leads to necrosis. Many infections asymptomatic; signs when appear include fever, depression, anorexia, vomiting, diarrhea, hemorrhages, and jaundice. “Blue-eye” occurs during convalescence – due to immune complex formation; can also affect the kidney |
|
|
|
what virus is associated with blue eye
|
canine adenovirus 1
|
|
|
|
canine adenovirus 2
|
Kennel cough
Replicates in respiratory tract epithelia. Antigenically similar to CAV 1 Used in vaccine. Upper resp tract |
|
|
|
bovine adenovirus
|
Bovine, ovine, and caprine serotypes.
At least 9 serotypes of bovine adenovirus. Virus replicates in epithelia of URT, LRT, conjunctiva, intestines. Pneumonia in calves with fever, ocular and nasal discharge, dyspnea and cough. Pneumoenteritis Respiratory and enteric disease in calves. Mainly resp disease - Can go to lower resp tract - Can survive transit in stomach and go to intestines but more common is resp disease |
|
|
|
equine adenovirus
|
Aerosol spread.
Most infections are self-limiting and asymptomatic. May present as mild respiratory tract disease with fever, nasal discharge, and cough. In immunocompromised foals, infection is progressive, leading to pneumonia and death. See in Arab foals with SCID. Widespread destruction of epithelial cells. |
|
|
|
group 1 aviadenovirus
|
conventional agents
Chickens, quail, ducks, pigeons. Inclusion body hepatitis. - Usually seen in broilers, 3-7 weeks of age. - Significant mortality Quail bronchitis. - Respiratory distress, cough, sneezing, ocular discharge. - Mortality 100% in young birds - Highly contagious, spreads rapidly. |
|
|
|
group II adenovirus (siadenovirus)
|
Hemorrhagic enteritis of turkeys.
- Turkeys older than 4 weeks of age; ~6-12 weeks. - Splenomegaly, intestinal hemorrhage. - Depression, bloody droppings; immunosuppression occurs. - May have significant mortality in affected flocks – up to 60%. Marble spleen disease of pheasants. - Despite its name, causes acute respiratory disease. - Seen in birds over 3 months of age. - Pulmonary edema leads to asphyxiation. Both siadenoviruses have characteristic splenic lesions. Viral-induced anaphylactic reaction. |
|
|
|
what virus is associated with egg drop syndrome
|
atadenovirus
|
|
|
|
atadenovirus
|
egg drop syndrome
Layers, other fowl, wild and domestic. Virus replicates in oviduct leading to necrosis of epithelial cells. Thin-shelled, soft-shelled, or shell-less eggs. Transmitted via eggs and droppings. |
|
|
|
adenovirus dx, tx, control
|
Diagnose by serology, virus identification using virus isolation or antigen detection assays. PCR becoming more widely available.
Treatment is supportive. Vaccines routinely used for canine adenovirus – uses CAV-2 to avoid “blue eye”. Human adenovirus not routinely vax for due to concern over oncogenesis; vaccine used in military recruits at high risk. |
|

