![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Two or more different atoms are combined in definite proportions in any? |
Element |
|
|
A covalent bond is formed by the |
Transfer of electrons |
|
|
When you shake sugar and sand together in a test tube, you cause them to form a |
Mixture |
|
|
A compound the produces hydrogen ions in a solution is a (n) |
Acid |
|
|
Compared to most other substances, a great deal of heat is needed to raise the temperature of water amount. This is because water |
Readily forms solutions |
|
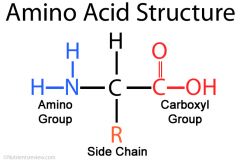
What does the following formula represent? |
An amino acid |
|
|
Proteins are polymers formed from |
Amino Acids |
|
|
An enzyme speeds up a reaction by |
Lowering the activation energy |
|
|
In a chemical reaction, a reactant binds to an enzyme at a region known as the |
active site |
|
|
Explain the relationship among atoms, elements, and compounds. |
Atoms make up elements, elements make up compounds |
|
|
What is a radioactive isotope? Describe two scientific uses of radioactive isotopes. |
Isotope has an unstable nucleus that decomposes by emission of a nucleus or helium nucleus and radiation Medicine and Industry |
|
|
Describe how the atoms in a compound are held together |
Chemical bonds. The interaction between the electrons forms. |
|
|
Explain the properties of cohesion and adhesion. Give an example of each property. |
Cohesive- attached to each other Adhesive- attached to a different element entirely. |
|
|
What is the relationship among solutions, solutes, and solvents? |
Solution- mixture in which one substance is dissolved in another. Solute- substance that is dissolved Solvent- substance in which the solute is dissolve |
|
|
How are acids and bases different. How do their PH values differ? |
Acids have more hydrogen ions (H+) than hydroxide ions (OH-) and have a pH of below 7. Bases have more hydroxide ions (OH-) than hydrogen ions (H+) and have a pH above 7. The lower the pH, the greater the acidity |
|
|
Identify the major functions of nucleic acids |
DNA & RNA |
|
|
Describe the two types of energy changes that can occur in a chemical reaction. |
Endothermic- molecules that require energy to break bonds. Exthermic- molecules or atoms often form bigger molecules and release energy. |
|
|
What relationship exists between an enzyme and a catalyst? |
Enzyme is a biological catalyst. An enzyme is a specific variable catalyst. |
|
|
Describe some facts that may influence enzyme activity |
Temperature pH,and regulatory molecules all affect enzymes |

