![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
71 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Whst is stronger. Sigma or pi bond? What orbitals are each? Pi vs signs Rotation Straight Stability Reactivity Big molecules are ___ than small molecules |

|
|
|
|
Hybridization Def How to determine? |

|
|
|
|
Angle and name for sp,sp2, 3, 3d, 3d2. And shape? |

|
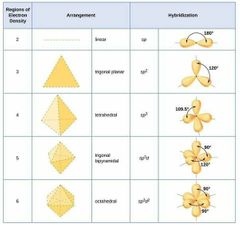
|
|
|
Electronegativity Trend H,c .. most and least, What's a dipole moment |

|
|
|
|
Energy ( break and form bonds) Heat of combustion Coordinate covalent bonds |

Break is released. Energy when formed |
|
|
|
Examples that break octet rule Valance Formal charge equation |

|
|
|
|
Ester vs ether |
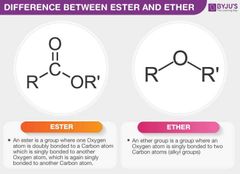
|
|
|
|
Alkoxy Alkyl Aldedyhe |
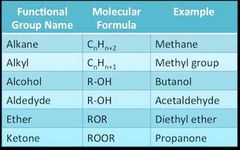
O-R alkoxy |
|
|
|
Gem |
|
|
|
|
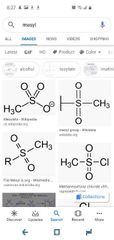
Mesyl |
|
|
|
|
Hydrazine Vinyl Allyl Nitryl |
H2NNH2 C=C C=C-C N=_H |
|
|
|
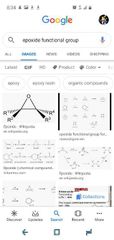
Exsposode |
|
|
|
|
Acyl Aliphatic Sulphone Hemiacetal |
R - C=O C-c-c-c O=S=O R - o -c- oh |
|
|
|
Iupac nomenclature 1-10 |

|
|
|
|
Isomer def Predict isomer formula (formula) Conformational Structural
|

|
|
|
|
Steroisomers Enantiomers vs diasteromers Chirality R and S. How figure out? Relative configuration ? |

|

|
|
|
Relative configuration Rotation of plane polarized light Clockwise vs counterclockwise |

|
|
|
|
Enatiomers -r v s - polarized light rotation? - ? |

|
|
|
|
Diasteromers E/z Cis vs trans Steric hinderance Epimers and anomers |

|
|
|
|
Base Nucliophoke Electrofile Leaving group |

|
|
|
|
E1, e2, sn1, sn2 |

|
|
|
|
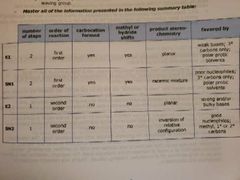
E1, e2, sn1, sn2 Step number, order, carbocation formed, methyl shift, steriochemkstry , favored by |
|
|
|
|
Alkane (chain leght/branching) Melting and boiling trends Lights
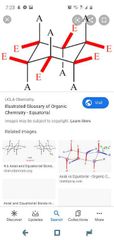
Ring strain? Axial vs equatorial |

|
|
|
|
Combustion formula General concept of radicals |

|
|
|
|
Syn and anti addition. Molecule responsible for this? |

|
|
|
|
Alkene Nueckiphiles? Stability? ( subs) Alkynes Benzene cs phenyl |

|
|
|
|
Alcahols Nomenclature (like icuap) Melting and boiling? Hydrogen bonding species? Alchohol acidity increases with? |

|
|
|
|
Oxidation of an alcohol Primary, secondary, 3 degree Common oxidation agents |

|
|
|
|
Reduction synthesis LiAlH4 vs NaBH4 Pinacol rearrangement |

|
|
|
|
Dehydration of alchohol Favored in hot vs cold. Concentrated vs dulte Grignard synthesis |

|
|
|
|
Ethers Definition Properties Most acts as an ? |

|
|
|
|
Epoxies |

|
|
|
|
Electronegativity in sn2 and sn1 predictions |

|
|
|
|
Electron donating and withdrawing groups Which determines which? |

|
|
|
|
Sn1 sn2, e1 e2 mechanism |

|
|
|
|
Acidity Why steric hinderance favors bases Why steric hinderance favors bases Why steric hinderance favors bases |

|
|
|
|
Electropholocity Predicting the product 1. Carbocation 2. Steric hinderance 3. Count the carbons |

|
|
|
|
How IR spectroscopy works? Vibration Frequency determined by 2? Absorbance if C=0 Oh Ch Carboxil oh Anime Amide Nitrile ( c=-N) |

|
|
|
|
How Uv spectroscopy works? 1 bond 2 or 3 bind Conjugate system? Graph is blank vs blank? |

|

|
|
|
Mass spect How it works? Parent peak vs base peak |

|

|
|
|
Nuclear magnetic resonance (c-nmr) Peaks Spin spin splitting? Area under peak? Absorbance range means? Shielding? |

|

|
|
|
CNMR and graph look? Dif in spin and integration Absorbance range? C-c C-o C=c C=O |

|
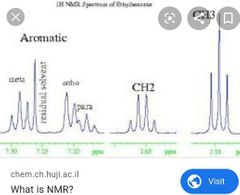
|
|
|
Extraction purpose of two compounds? Separation depends on? How it works? |

|
|
|
|
Improving separation techniques ? 4? 3 things to avoid. Y? |

|
|
|
|
Gravity filtration Vacuum filtration ? Distillation? - simple and fractured? - vacume distillation? |

|
|
|
|
Chromatography What is it? 2 phases? First substance out is? Rf<1 vs 1<
Paper chromo ? |

|

|
|
|
Column, ion, and affinity chromo? And gas? |

|

|
|
|
Christalization work? Well? |

|
|
|
|
Carbonyl function group? Def? Properties (compare to alkene?) 5 key features ? Hint: (charge, alpha, eltrons, hinderane, aterochemisty) |

|
|
|
|
Importance of alpha hydrogen? Acidity of alpha |

|
|
|
|
Aldehydes and ketones Definition Nomenclature Some common names? Solubility and boiling point trends? H bonds ? |

|

|
|
|
General chatectinces if aldehyde/ketone Major function? Sub vs addition ? |

|
|
|
|
Keto-enol tautomerization |

|
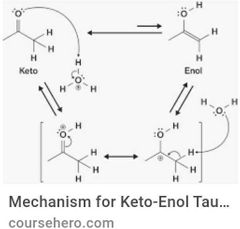
|
|
|
Acetals/hemiacetals and ketals/hemiketals formation. 4 steps and mechanism |

|
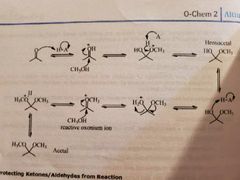
|
|
|
Protecting ketones and aldehydes Halogenation if aldehydes or ketones |

|
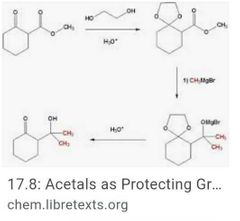
|
|
|
Aldol condensation 3? Word of caution? |
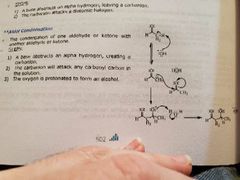
|

|
|
|
a-B unsaturated carbonyl ( how to turn carbonyl to alchohol ) |

|
|
|
|
Carboxylic acid Def Nomenclature- common Physical properties (boil and solubility) |

COOH |
|
|
|
Carboxylic acid 3 gen properties Nicleopholic attack of carbonyls Decarboxilation ? Esterification (pic is later) |

|
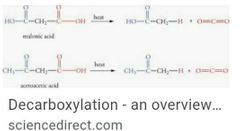
|
|
|
Acid chloride Def Nomenclature Formation and 3 main carboxylic acids Most reactive to what? |

|

|
|
|
Anhydrides Def Nomenclature Properties |

|
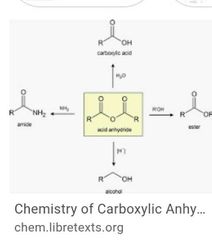
|
|
|
Amides Def Nomenclature Stability? How does resonance limit rotation? |

|
|
|
|
Esters ? Def Nomenclature Properties Transesterfication Saponification |

|
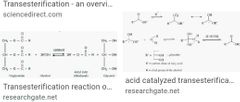
|
|
|
Acetoacetic ester synthesis Forms what from what? 3 steps? Inorganic esters? |
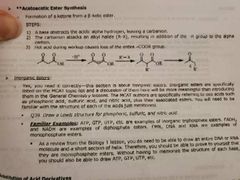
|
|
|
|
Substitution of acid derivative? Mechanism and leaving groups? Stability if carboxylic acid derivatives? 5 |

|
|
|
|
Amines ? Def? Base or nucleophile? Depends on was what? Basicity? Nomenclature? |

|
|
|
|
Synthesis if alkyl amines? Gabriel synthesis? |

|
|
|
|
Reduction synthesis of amines? Main two agents ? Nitro Nitrile Imines Amides |

|
|
|
|
Addition of amines to carbonyls? 3 steps Primary Seci dart Tertiary? |

|
|
|
|
Easterification |
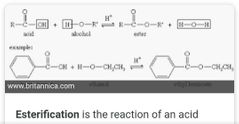
|
|
|
|
Acedic and formic acid |
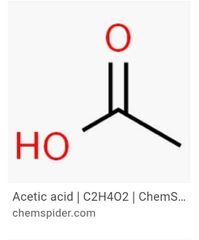
|
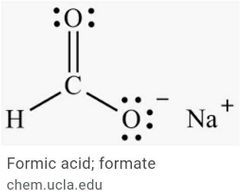
|

