![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
62 Cards in this Set
- Front
- Back
|
T-Butyl Chloride: After the reaction why was 5% aqueous sodium carbonate added to the organic layer?
|
To neutralize the acid
|
|
|
T-Butyl Chloride: Why was anhydrous magnesium sulfate used?
|
To dry the product before measuring the IR
|
|
|
Cyclohexanone: What IR Peak is gone from the product but was present in the reactant?
|
OH
|
|
|
Cyclohexanone: What new IR peak would appear in the product but was absent in the reactant?
|
C=O
|
|
|
Cyclohexanol: How does the IR Spectrum of the starting cyclohexanone change when product cyclohexanol forms?
|
There is a loss of the C=O peak and formation of the OH peak.
|
|
|
Cyclohexanol: What substance is extracted into the ether layer after the reaction is over?
|
Cyclohexanol
|
|
|
Cyclohexanol: Why was anhydrous magnesium sulfate used?
|
To dry the product
|
|
|
Diels- Alder Reaction: What is the purpose of heating the dicyclopentadiene before addition of maleic anhydride?
|
To prevent the cylopentadiene from dimerizing.
|
|
|
Diels-Alder Reaction: What happens to the cyclopentadiene if it is allowed to stand too long?
|
If left to stand, cyslopentadiene will dimerize back to dicylopentadiene.
|
|
|
Methyl Nitrobenzoate: Why is sulfuric acid used?
|
As a catalyst
|
|
|
Methyl Nitrobenzoate: Why is the product washed with water?
|
To remove acid
|
|
|
Methyl Nitrobenzoate: Why is the product washed with methanol?
|
To remove the more soluble ortho isomer.
|
|
|
Bromination of Acetanilide: Besides bromination at the para position, what small amount of other product might form when Br2 reacts with acetanilide?
|
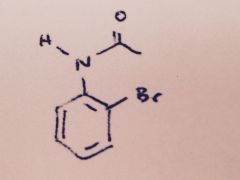
|
|
|
Bromination of Acetanilide: After the reaction, sodium bisulfite is added to react with what leftover substance?
|
xs Br2
|
|
|
Grignard Reaction: How will the IR spectrum of alcohol product differ from the ketone reactant?
|
Noticeable OH peak and absence of C=O peak
|
|
|
Grignard Reaction: The glassware and reagents must be water free. With what substance will water react resulting in an unwanted side reaction?
|
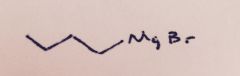
|
|
|
Grignard Reaction: What substance is extracted into the ether layer?
|
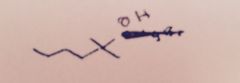
|
|
|
Why is a solvent containing a solid product often cooled before filtering to obtain the solid product?
|
To allow for formation of any remaining product.
|
|
|
After filtering on a Buchner funnel, why is the solid washed with a cold solvent?
|
To remove impurities (i.e isomers)
|
|
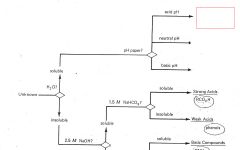
|
RCO2H
|
|
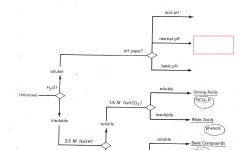
|
ROH
RCHO RCN |
|
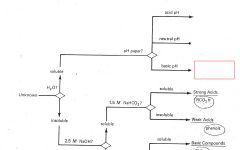
|
RNH2
R3N |
|
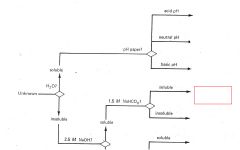
|
RCO2H
|
|
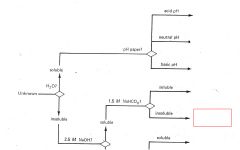
|
Phenols
|
|
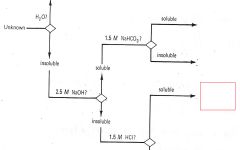
|
RNH2
R3N |
|
|
ROH
|
|
|
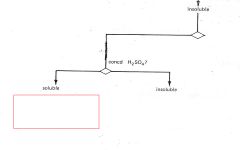
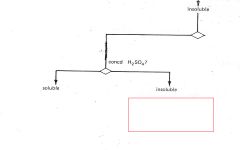
Saturated Hydrocarbons
|
|
|
Round Bottom Flask
|
|
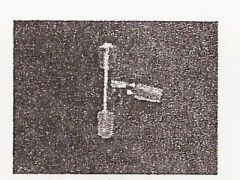
|
Y Adaptor
|
|

|
Vacuum Adaptor
|
|
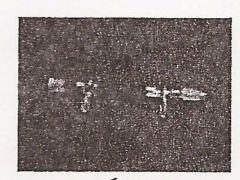
|
Condenser
|
|
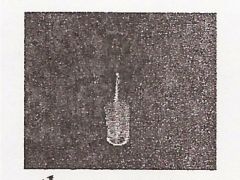
|
Thermometer Adaptor
|
|
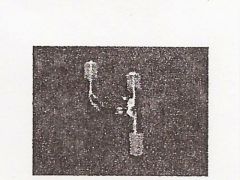
|
Claisen Adaptor
|
|

|
Beaker
|
|
|
Erlenmeyer Flask
|
|

|
Filtering Funnels
|
|

|
Vacuum Flask
|
|
|
Crystallizing Dish
|
|
|
Separatory Funnel
|
|

|
Stir Rod
|
|

|
Thermometer
|
|
|
Graduated Cylinders
|
|

|
Watch Glass
|
|
|
Test Tubes
|
|
|
KMnO4
|
|
|
RCOOH
|
|

|
Loss of purple color
|
|
|
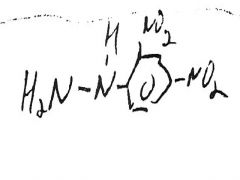
|
|
|
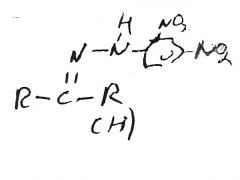
|
|
|
Red/Yellow ppt.
|
|
|
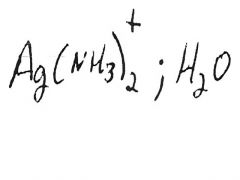
|
|
|
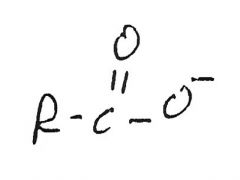
|
|
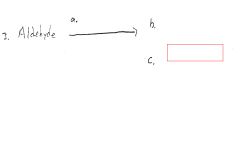
|
Silver mirror or black ppt
|
|
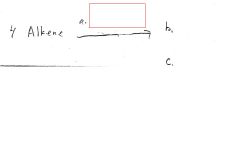
|
Br2/CCl4
|
|
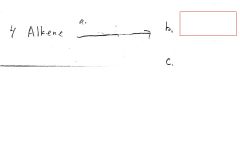
|
Br-C-C-Br
|
|
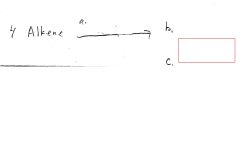
|
Loss of red/brown color
|
|
|
Fe+3
|
|
|
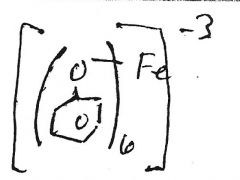
|
|
|
red/blue/purple/green solution
|
|
|
|
|
|
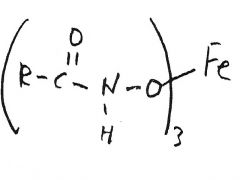
H2NOH-HCl
________________ Fe+3 |
|
|
red-violet solution
|

