![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
- 3rd side (hint)

|

|
|
|

|

|
|
|

|

|
|
|
|
CH3-NH2
|
methylamine
(methanamine) |
|
|
|
CH3-CH-CH2-NH2
| CH3 |
isobutylamine
(2-methyl-1-propanamine) |
|
|
|
(C2H5)3N
|
triethylamine
(N,N-diethyl-ethanamine) |
|
|
|
CH3-CH2-CH2-N-CH3
| C2H5 |
ethylmethylpropylamine |
|
|
|
Cl-CH2-CH2-NH2
|
2-chloro-ethylamine
(2-chloro-ethanamine) |
|
|
|
pKas of NH3 CH3NH2 CH3CH2NH2 CH3CH2CH2NH2
|
9.26 10.64 10.75 10.67
|
|
|
|
basicity of NH3, 1°, 2°, 3°
|
2° > 1° > 3° > NH3 |
|
|

name
|
aniline
|
|
|
|
which is more basic? |
electron donating (-CH3, -NH2, -OCH3) will increase reactivity of the ring and make it more basic compared to electronic withdrawing (-NO2, -Cl, -CN) |
|
|
|
CH3CH2CH2-NH2 + CH3-Br -> ?
|
R-NH-R' (2° amine)
|
|
|
|
Gabriel synthesis |

|
|
|

Friedel Crafts Alkylation |
BENZENE-C(CH3)3
|
|
|
|
Friedel Crafts Alkylation Limitations |
1. only alkyl halides work. not aryl or vinyl (double bond) halides |
|
|

|

|
|
|
|
Friedel Crafts Acylation Limitations |
1. Multiple substitutions DO NOT occur |
|
|

|

|
|
|
|
Canizzaro Reaction |
benzaldehye is attacked by base (-OH) and one benzoic acid (carboxylic acid) and one benzyl alcohol is formed |
2 benzaldehyes without an alpha H
|
|

|

|
|
|

|
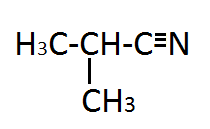
|
|
|

|

|
|
|

|

|
|

