![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Three classes of neurotransmitters
|
Small molecule, neuroactive peptides and gaseous
|
|
|
Small molecule Transmitters:
• 3 groups • Place of synthesis • Site of storage |
Small Molecule Transmitters:
• Acetylcholine, Excitatory AAs and Biogenic amines • in the cytosol of nerve terminals • After being taken up by empty clear vesicles, they will be tethered to the cytoskeleton until release into the active zone |
|
|
Filling a synaptic membrane depends on this
|
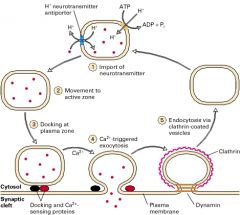
An established H+ gradient across the vesicular membrane
Typically, An (H+)-ATPase pumps H+ into the cell while an antiport shuttles an H+ out of the cell in exchange for a desired NT |
|
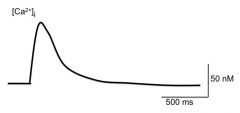
Briefly describe what accounts for the rapid rising phase (1) and slow falling phase of intracellular [Ca2+]
|
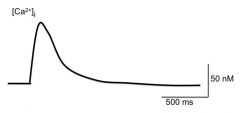
Rising phase: attributed to the AP. The Ca2+ influx is needed for exocytosis
Falling phase: membrane channels are either pumping out Ca2+ or sequestering it from the cytosol On the plasma membrane of the terminal nerve ending are a (Ca2+)-ATPase and a Ca2+/Na+ exchanger In the cytosol is the Sarcoplasmic reticulum which sequester Ca2+ using SER-Ca2+ ATPase. |
|
|
Exocytosis of of NTs:
• Role of Synapsin • What causes Synapsin to be Phosphorylated? • Role of Rab proteins • Which proteins are involved in docking of the vesicle to the plasma membrane? (4) • Which protein helps the vesicle fuse with the nerve terminal membrane? • What is needed for the vesicle to fuse with the membrane? |
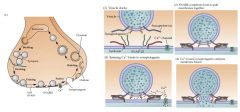
Exocytosis of of NTs:
• To tether the vesicle to the cytoskeleton • Rising [Ca2+]i • brings the vesicles to the active zone for exocytosis • Two t-SNARE and Two v-SNARE proteins are needed. The t-Snare proteins, which are located on the plasma membrane, are Syntaxin and Synaptobrevin. They bind, respectively, to two v-SNARE proteins, Synaptobrevin and Synaptotagmin. • Synaptophysin • A local rise in [Ca2+]i at the active zone |
|
|
This determines the release of small molecule NTs vs that of small molecule NTs AND neuropeptides
|
Small molecule NTs are stored in small clear vesicles while Neuropeptides are stored in large dense core vesicles.
What determines their release are stimulation rates: - Low nerve stimulation rates (< 10Hz) small clear vesicles are preferentially released - High nerve stimulation rates (>100Hz) induces both small and large core vesicles to be released Remember that the stiulation rates are dependent on the level of [Ca2+]i |
|
|
Vesicular Membrane Recycling:
• General process by which vesicles are recovered • For small clear vesicles, - this protein mediates its recycling - once inside, it fuses with this structure • For dense core vesicles, once they get back into the nerve terminal, - they are transported to ....... via this process - the above process is driven by which protein? - the vesicles are filled with....... |
Vesicular Membrane Recycling:
• General process by which vesicles are recovered • For small clear vesicles, - clathrin - endosome • For dense core vesicles, once they get back into the nerve terminal, - ... to the soma via retrograde transport - dynein - ... filled with propeptides |
|
|
Miniature end plate potentials:
• The means by which they were induced • Their existence showed this |
minEPPs
• They were not induced, but were spontaneously generated in the ansence of nerve stimulation • Their existence showed that the nerve terminal is releasing ACh spontaneously |
|
|
What did replacing Ca2+ with Mg2+ do to EPPs? (2)
|
• It lowered the probability of a vesicle being released via exocytosis
• It lowered the value of EPPs, showing quantal fluctuations as all EPPs are multiples of minEPPs |
|
|
Def of Quantal size
|
The number of transmitter molecules in a vesicle
|
|
|
Def of Quantum Content
|
The number of Quanta released by a single impulse
|
|
|
Botulinum toxin:
• Its mechanism of action • What it prevents • A clinical use |
Botulinum toxin:
• It is a protease that degrades synaptobrevin, preventing the vesicle from docking • It irreversibly prevents ACh release • Tx of dystonia, irreg clonic contractions of muscle |
|
|
Tetanus Toxin:
• Its target cell • Its mechanism of action • What it prevents |
Tetanus Toxin:
• Glycinergic interneurons • It is a protease that degrades synaptobrevin • It prevents vesicular release of glycine irreversibly |
|
|
α-Latrotoxin:
• Type of molecule • Its mode of action • The result of its actions |
α-Latrotoxin:
• peptide • It binds to the neurexin/synaptotagmin promoting fusion of cholinergic vesicles • After the large release of vesicular ACh, the temrminal becomes depleted of vesicles leading to spasms and succeeding flaccid paralysis |
|
|
Aminoglycoside Antibiotics:
• two examples • mode of action • How the effects of the Abs can be reversed • What they do at the NMJ at high concentrations |
Aminoglycoside Antibiotics:
• Neomycin and Streptomycin • inhibit exocytosis at the nerve terminal • Their effects are reversed competitively by raising [Ca2+] levels • They can block nAChR at high concentrations |
|
|
4-Aminopyridine:
• Mode of action (2) • The probability of this is increased |
4-Aminopyridine:
• It blocks K+ channels, prolonging the duration of an impulse (preventing re-polarization) and enhancing Ca2+ into the motor nerve ending • The probability of ACh release is increased (Quantum Content is increased) |
|
|
These kinds of synapses can lead to neurotransmitters altering Quantal release
|
Axo-axonic synapses; the transmitters released from the pre-synaptic cell bind to the receptors on the axon of the post-synaptic cell, inhibiting/reducing the release of its NTs
|
|
|
Hypocalcemia:
• a disease that causes it • Three consequences of it |
Hypocalcemia:
• Hypoparathyroidism • Three consequences 1) The lack of Ca2+ in the ECF lowers the membrane potential gradient btwn the inside and outside of the cell, lowering the threshold for excitability, making it more likely to spontaneously depolarize 2) Lower Ca2+ makes the plasma membrane more permeable to ions and small solutes, also leading to depolarization 3) However, the lack of Ca2+ will also lead to less transmitter/quanta released from the pre-synaptic terminal |
|
|
β-bungarotoxin
• what it binds to • the end result |
β-bungarotoxin
• binds to actin irreversibly • Blocks ACh release |

