![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
Common organic elements |
Hydrogen, Oxygen, Carbon, Nitrogen |
|
|
Hydrophobic |
Will not interact with water, eg: fatty acid |
|
|
Hydrophilic |
Affinity for and interacts with water, eg: salts, Vitamin C |
|
|
Amphipathic |
Molecule that is part hydrophobic and part hydrophilic |
|
|
Cohesion (water) |
Formation of hydrogen bonds with other water molecules |
|
|
Adhesion (water) |
Polarity of water makes it attracted to other molecules, allows substances to dissolve easily |
|
|
Other key properties of water: |
- High specific heat (can store heat) - High latent heat of vaporisation (takes a lot of energy for phase change) - High density |
|
|
Comparing thermal properties of water and methane |
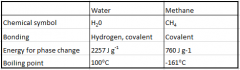
|
|
|
Water (heat transport) |
- Plasma is 55% of blood and composed of 92% water |
|
|
Water (habitat) |
- High specific heat - Takes a long time to heat up or cool large bodies of water, making it stable |
|
|
Water (coolant) |
- High specific heat and latent heat of vaporization - Evaporation causes hydrogen bonds to break apart, using energy - Sweat allows cooling as heat energy is expended to break H-bonds |
|
|
Water (transport in plants) |
- Adhesion: water molecules attracted to surface of xylem vessel wall, creates high adhesion forces - Cohesion: water molecules evaporate from surface of a leaf cell, others are pulled from behind creating a transpiration stream
|
|
|
Monosaccharides (3) |
- Glucose |
|
|
Disaccharides (3) |
- Maltose (glucose x2) - Lactose (glucose + galactose) - Sucrose (glucose + fructose) |
|
|
Draw Ribose |
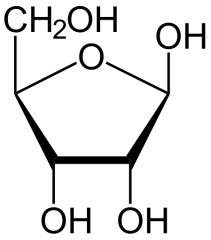
|
|
|
Alpha-D-glucose and Beta-D-glucose |
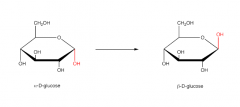
|
|
|
Polysaccharides |
More than two monosaccharides joined to form a chain: starch, cellulose, glycogen |
|
|
Cellulose structure |
- beta-D-glucose - 1-4 linkage - Unbranched, straight chains - Very strong due to cross-linkages by hydrogen bonding |
|
|
Starch structure |
- alpha-D-glucose - 1-4 linkage - Two forms: amylose and amylopectin - Amylose unbranched - Amylopectin is slightly branched: 1-6 linkages - Both forms coiled and insoluble |
|
|
Glycogen structure |
- alpha-D-glucose - 1-4 linkage - Profusely branched: 1-6 linkages - Insoluble |
|
|
Cellulose function |
- Cell wall: support, structure, protection - Porous, allows water through |
|
|
Starch function |
- Energy storage in plants - Both forms found together in starch grains |
|
|
Glycogen function |
- Energy storage in animals - Stored in liver, skeletal muscle cells - Forms granules in cytoplasm |
|
|
Lipids (3) |
- Triglycerides - Phospholipids - Steriods |
|
|
Triglyceride structure |
Glycerol + three fatty acids, formed by condensation |
|
|
Fatty acids: structure, types |
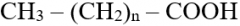
- Saturated: no double bonds, more energy - Unsaturated: one or more double bonds (mono + poly unsaturated) |
|
|
Cis vs. Trans |
- Cis: Hydrogen atoms on the same side of the double bond, come in omega-3 and omega-6 - Trans: Hydrogen atoms on opposite sides, partially or fully hydrogenated |
|
|
Phospholipids |
- Modified triglyceride - Third fatty acid replaced with phosphate group - Make the molecule polar |
|
|
Steroids |
- Three 6-sided rings, one 5-sided ring - Functional (F) group can be attached to 5-sided ring |
|
|
Carbohydrates as energy stores |
- 17 kJ per gram - Easily built up and broken down - Glycogen in animals, starch in plants: convert to glucose when energy is required |
|
|
Lipids as energy stores |
- 38 kJ per gram - More efficient because lipids are hydrophobic and less mass is taken up storing water - Break down more slowly - Converted into fatty acids and glycerol and then into Acetyl-CoA |
|
|
LDL & HDL |
- Low and high density lipoprotein, transport molecules for lipids - LDLs referred to as 'bad' cholesterol, HDLs 'good' cholesterol |
|
|
Condensation and hydrolysis in carbohydrates (draw) |
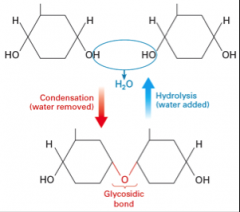
|
|
|
Condensation and hydrolysis in lipids (draw) |
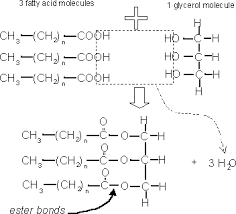
|
|
|
Condensation and hydrolysis in amino acids (draw) |
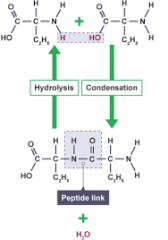
|
|
|
Residue |
Part of monomer lost during formation of a di/polymer |

